Calculate pH of Buffer Solution
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to learn how to determine the pH of a buffer solution via the Henderson-Hasselbach buffer equations.
Usually we are taught that there are 2 buffer equations for different buffer solutions.
We use the acidic buffer equation to determine the pH of an acidic buffer, which is a mixture of weak acid and salt of conjugate base.

We use the alkaline buffer equation to determine the pH of an alkaline buffer, which is a mixture of weak base and salt of conjugate acid.

But is it really true that an acidic buffer and alkaline buffer are 2 different types of solutions?
Both are just examples of a mixture of conjugate acid-base pair.
So if they are fundamentally the same type of solution, then the two buffer equations should be interchangeable.
Let's prove this via an example where we have a solution that contains 0.1 moldm-3 CH3COOH and 0.01 moldm-3 CH3COONa, given pKa of CH3COOH is 5.

A buffer is a mixture of a conjugate acid-base pair and in this case the acid will be CH3COOH while base will be CH3COO-.

1. Treat as Acidic Buffer
Since this is a mixture of a weak acid and conjugate base, the most direct way is to treat this as an acidic buffer and use the acidic buffer equation to determine the pH of the solution to be 4.

2. Treat as Alkaline Buffer
We can also use the alkaline buffer equation to calculate the pH but need to take note on the following points:
- pKb is for base CH3COO- which we can determine from pKa of CH3COOH via the formula
pKa + pKb = 14
- base is CH3COO- hence "salt" must be CH3COOH which is the conjugate acid partner of CH3COO-
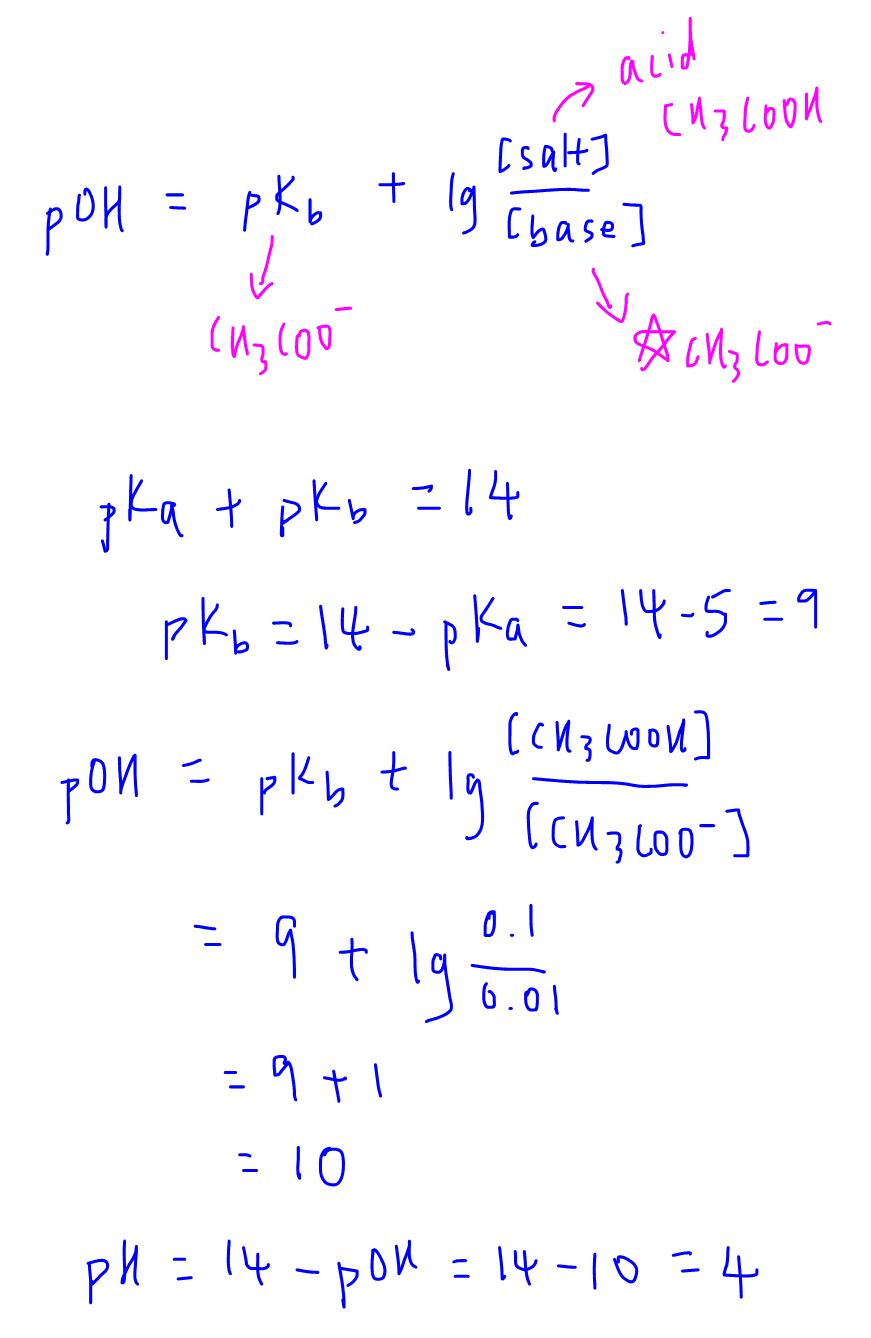
Interestingly the pH calculated using the alkaline buffer equation will give the same answer pH = 4.
3. Conclusion
This means that both equations can be used to calculate the pH of any buffer solution.
Personally I'll recommend students to use the acidic buffer equation if Ka is given, and the alkaline buffer equation if Kb is given.
This will allow us to determine pH in the shortest number of steps.

For the detailed step-by-step discussion on how to calculate the pH of any buffer solution, check out this video!
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
