Calculate pH of Salt Solution
In this video we want to deduce the nature and calculate the pH of sodium ethanoate solution.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this question to calculate the pH of 0.500 mol dm-3 sodium ethanoate given Ka of ethanoic acid is 1.8 x 10-5 mol dm-3.

Salt Hydrolysis
First we will have to deduce the nature of sodium ethanoate, which is a concept of salt hydrolysis.
I have a previous video on salt hydrolysis so if you are interested do check it out.
To summarise this is the concept that we need to know:
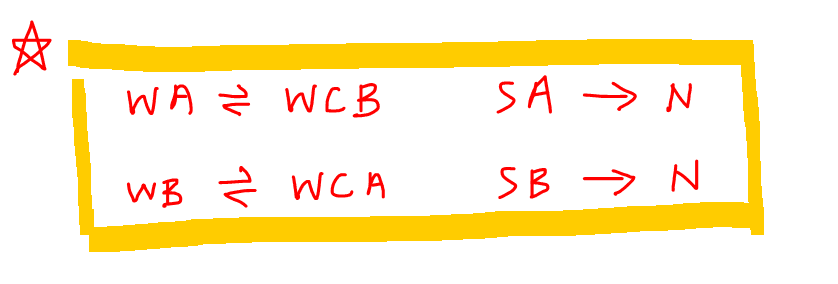
- weak acid (eg CH3COOH) will dissociate to give weak conjugate base (CH3COO-)
- weak base (eg NH3) will dissociate to give weak conjugate acid (NH4+)
- strong acid (eg HCl) will dissociate to give neutral counter ion (Cl-)
- strong base (eg NaOH) will dissociate to give neutral counter ion (Na+)

So for sodium ethanoate, we can deduce that CH3COO- is the conjugate base of weak acid CH3COOH, hence will dissociate in solution to give OH- and pH will be greater than 7.
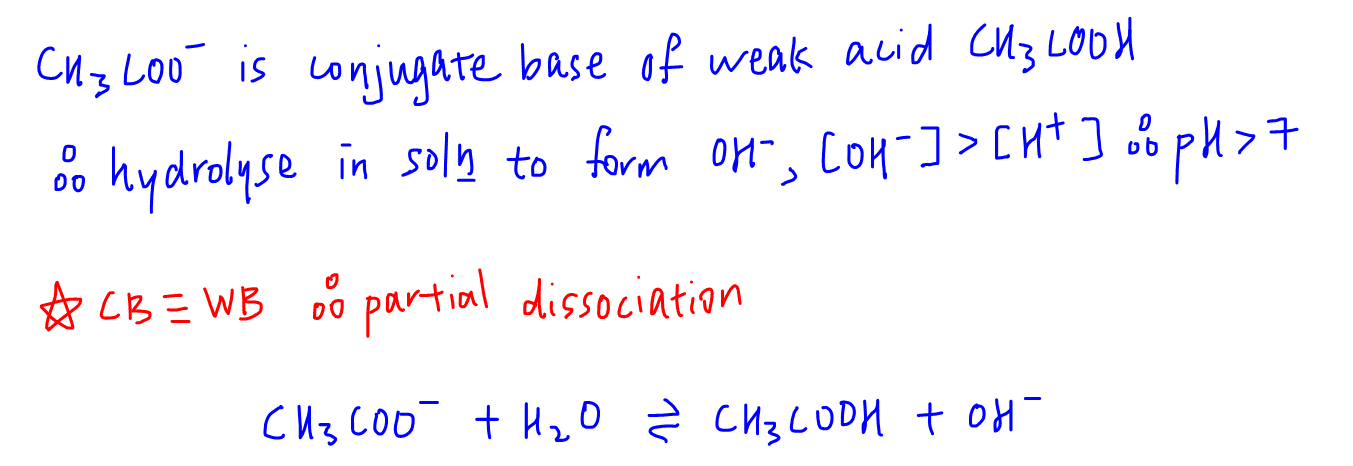
Notice the dissociation is reversible as conjugate base ethanoate is a weak base.
So for calculation of pH we can use the formula of weak base to determine the OH- concentration and eventually solve for pH.

Kb of ethanoate is not known but we can determine it from Ka of ethanoic acid using the formula:
Ka x Kb = Kw (for conjugate acid-base pair)
It is important to note that the method to find pH of a salt solution is to deduce that one of the ions is a conjugate base (in this example), which is also a weak base.
So the method to find the pH of sodium ethanoate is nothing more than finding the pH of a weak base.
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
