How to Calculate pH of Water
In this JC2 webinar we want to learn how to calculate the pH of water and deduce its nature at different temperatures.
1. Auto-ionisation of water
Pure water will undergo acid-base reaction with itself to a very small extent to form H+ and OH-.
This process is known as auto-ionisation of water and the concentration of H+ and OH- are equal since water will dissociate to give equimolar amounts of H+ and OH-.
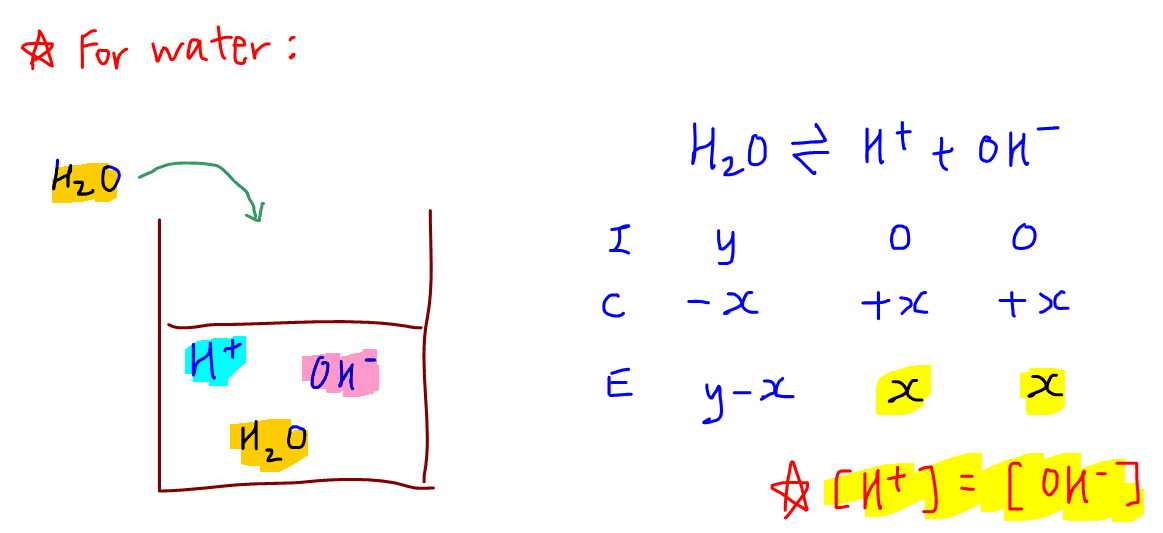
The equilibrium constant for this dissociation is known as the ionic product of water, Kw, where
Kw = [H+][OH-]
It has a given value of 10-14 mol2 dm-6 at 25oC or 298K.
We also need to know that auto-ionisation of water is an endothermic process.
2. Calculate pH of water at 298K
Since concentration of H+ and OH- in pure water are the same, we can calculate pH of water to be 7 via the following working:
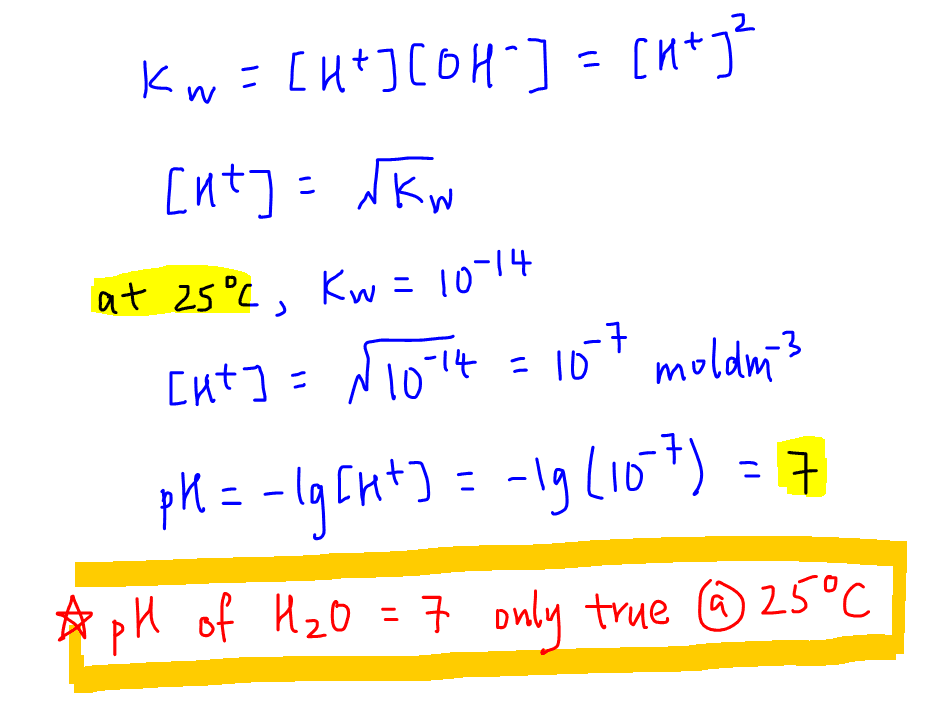
At first glance this seems hardly surprising, but pH of water is 7 only when Kw = 10-14 at 25oC.
When there is a change in temperature, Kw, H+ concentration and therefore pH will change.
3. Deduce pH of water when temperature is above 298K
Recall that auto-ionisation is endothermic.
When there is an increase in temperature, the position of equilibrium will shift right to favour endothermic reaction to absorb excess heat.
Since the forward reaction is favoured, more H+ and OH- is formed and their concentrations will increase.
This means H+ concentration will be greater than 10-7 mol dm-3, and the corresponding pH will be less than 7.
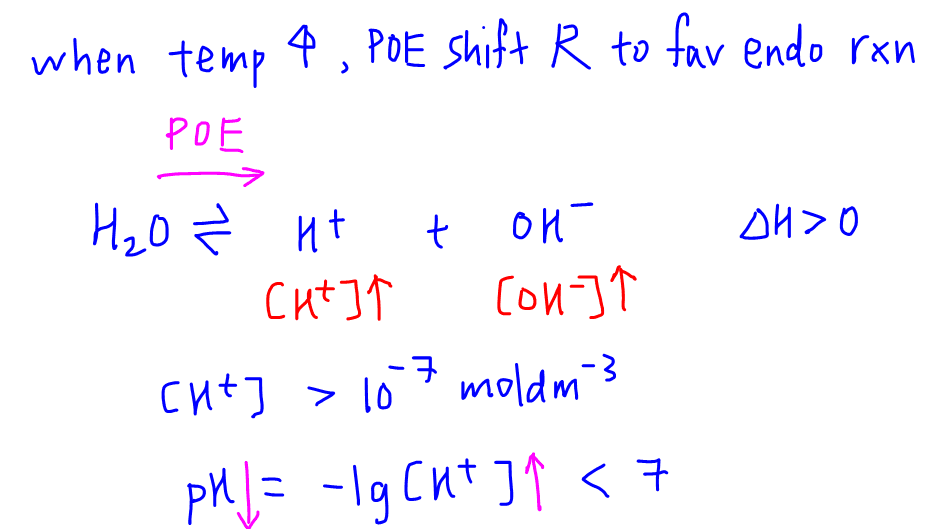
Hence pH of water will be less than 7 at temperatures above 298K, and greater than 7 at temperatures below 298K.
Does this mean that water becomes acidic when temperature is above 298K, and water becomes alkaline when temperature is below 298K?
4. Deduce nature of water at different temperatures
It is not possible to change the nature of water by changing its temperature.
At any temperature, regardless of the extent of dissociation, the concentration of H+ and OH- will always be the same.
This means for pure water, [H+] = [OH-] will always be true and water will always be neutral regardless of temperature.
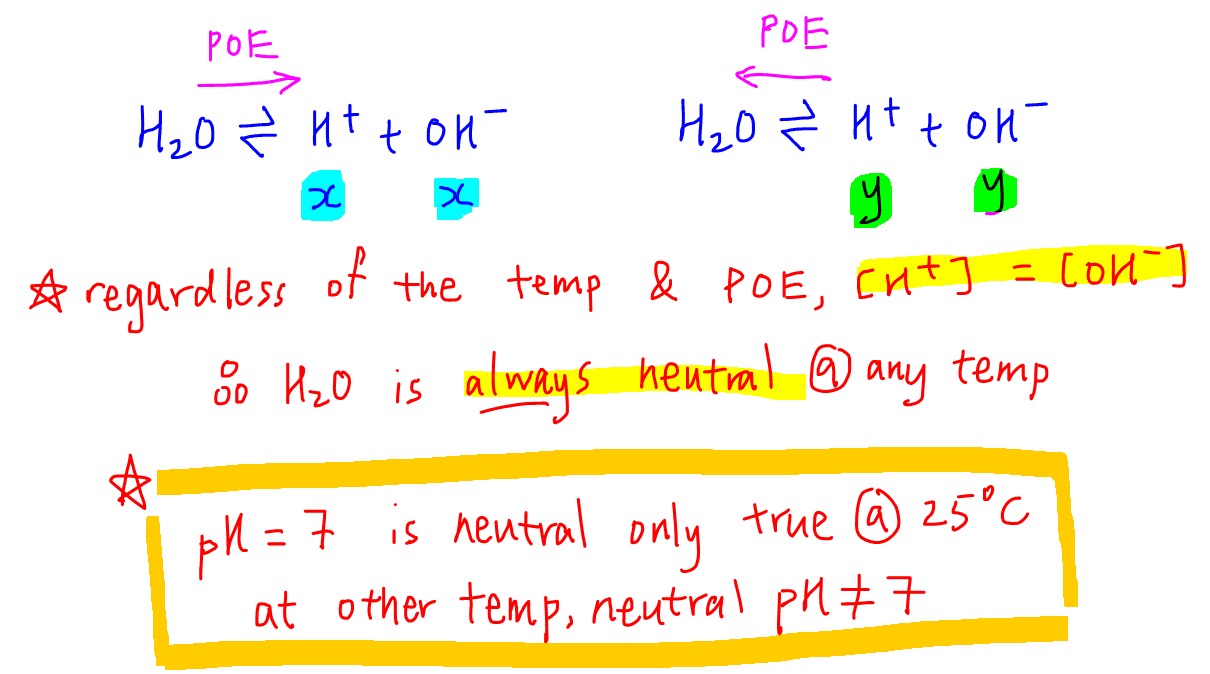
The reason why students might find this confusing is because we tend to use pH to deduce whether a solution is acidic, neutral or alkaline.
The concept that a solution is acidic when pH is less than 7, neutral when pH is 7, and alkaline when pH is greater than 7 is only true at 25oC or 298K.
When dealing with non-standard temperatures, we have to compare H+ and OH- concentrations to deduce the nature of solution:
Acidic when [H+] > [OH-]
Neutral when [H+] = [OH-]
Alkaline when [H+] < [OH-]
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
