How to Calculate pH of Weak Base
In this JC2 webinar we want to learn how to calculate the pH of a weak base.
The method is very similar to finding the pH of a weak acid.
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through the question.
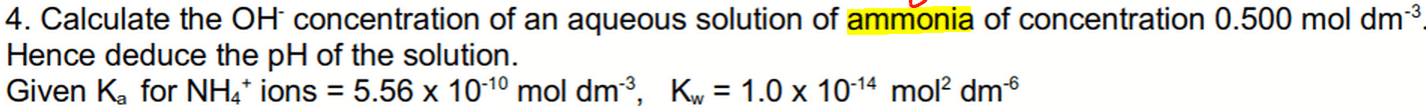
Since ammonia is a weak base, it will be partially dissociated.
We can work out the concentrations of all the species at equilibrium via the ICE table.
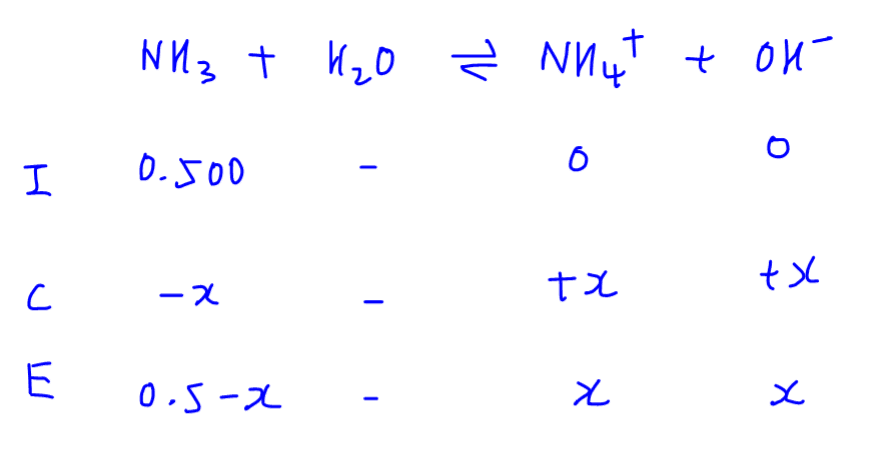
We can then substitute these values into the base dissociation constant (Kb) expression.
In order to solve for x, we need to impose an approximation to avoid solving quadratic equation.
We will assume that the concentration of weak base at equilibrium is equal to its original concentration.
We can then rearrange the equation to determine x, which is the concentration of OH-.
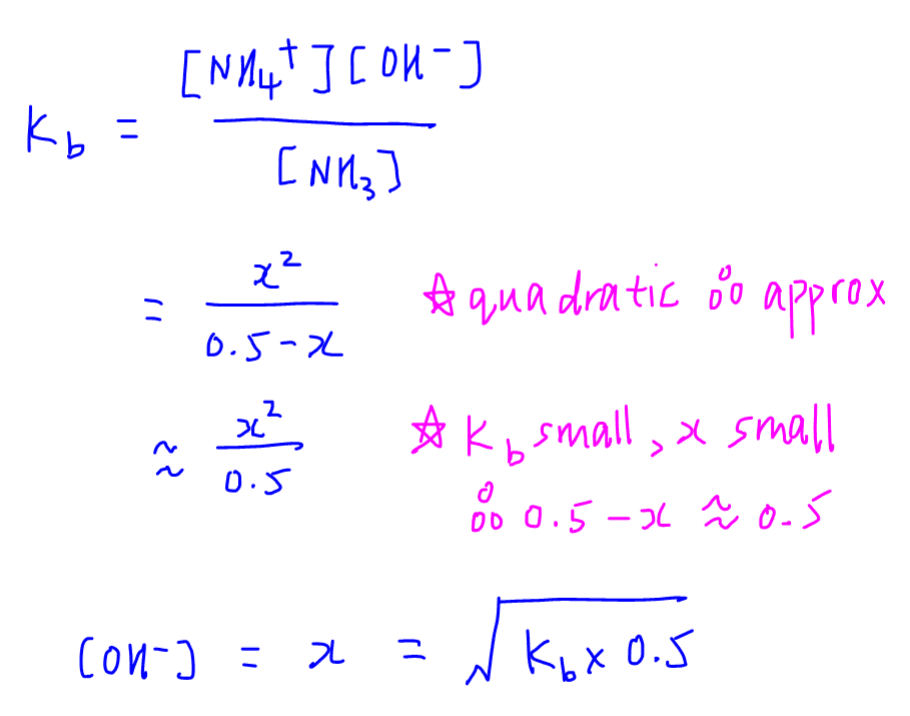
Since we are dealing mostly with monoprotic species in Ionic Equilibria, the ICE table and approximation is always valid.
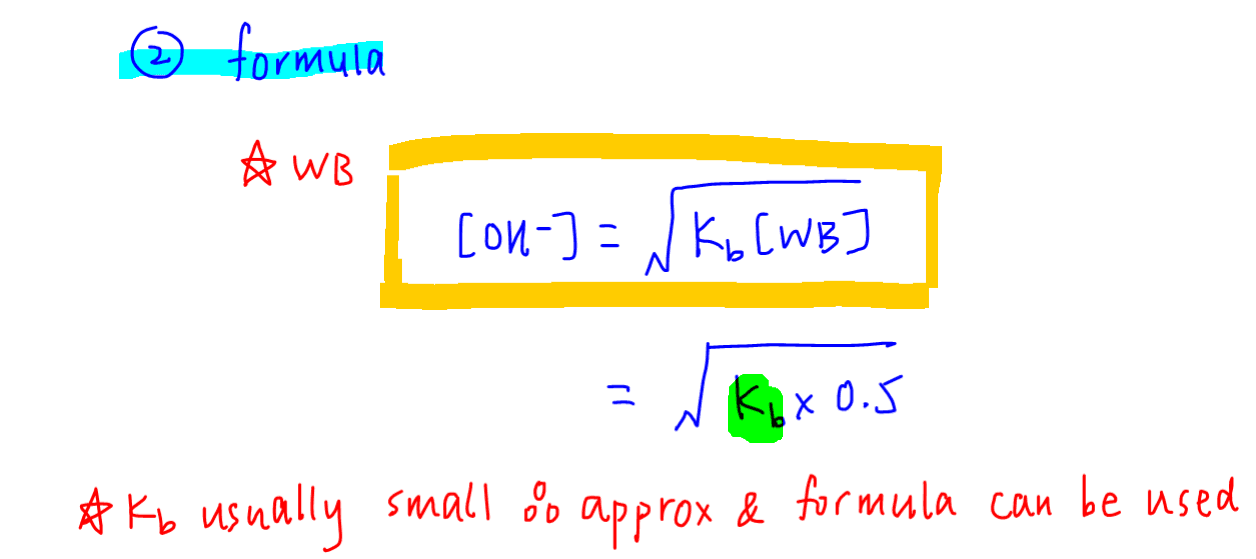
Therefore we can use the following formula to quickly determine OH- concentration when Kb and weak base concentration are given.
For this exercise Kb of ammonia is not given.
But we can get this easily from the Ka of its conjugate acid NH4+ and Kw, the ionic product of water.
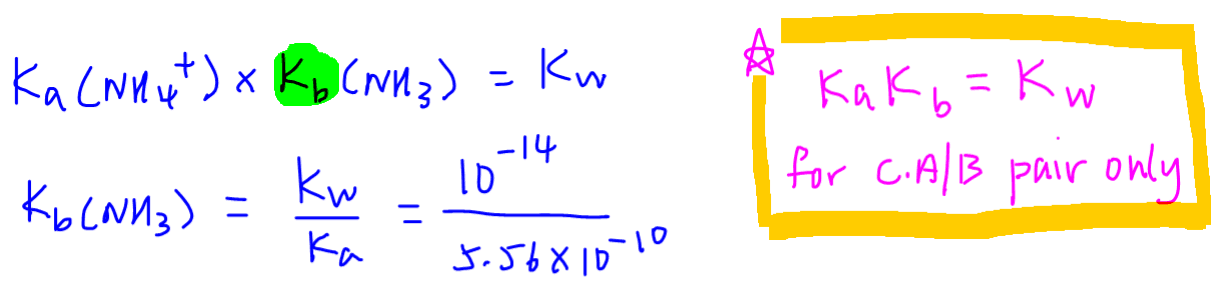
Please note Ka.Kb = Kw only applies to conjugate acid-base pairs.
We can now use the formula method to find OH- concentration.

Finally we can determine pOH and pH of this weak base.
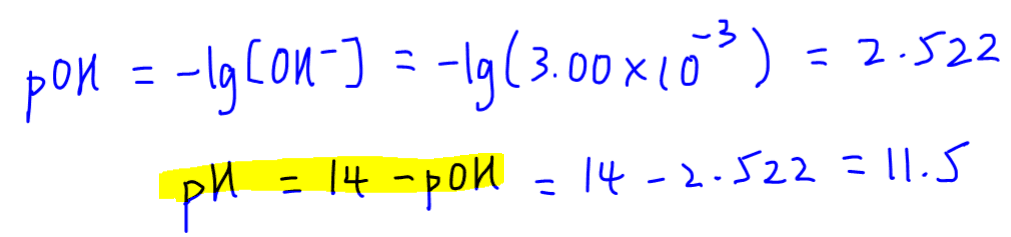
Topic: Ionic Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
