How to Calculate Solubility in Presence of Common Ion
Let Chemistry Guru, Singapore's esteemed A Level Chemistry tuition centre, guide you through our discussion question this week!

We want to determine solubility of iron (II) hydroxide in 0.10 moldm-3 of FeSO4.
Let's consider the dissociation of both salts.
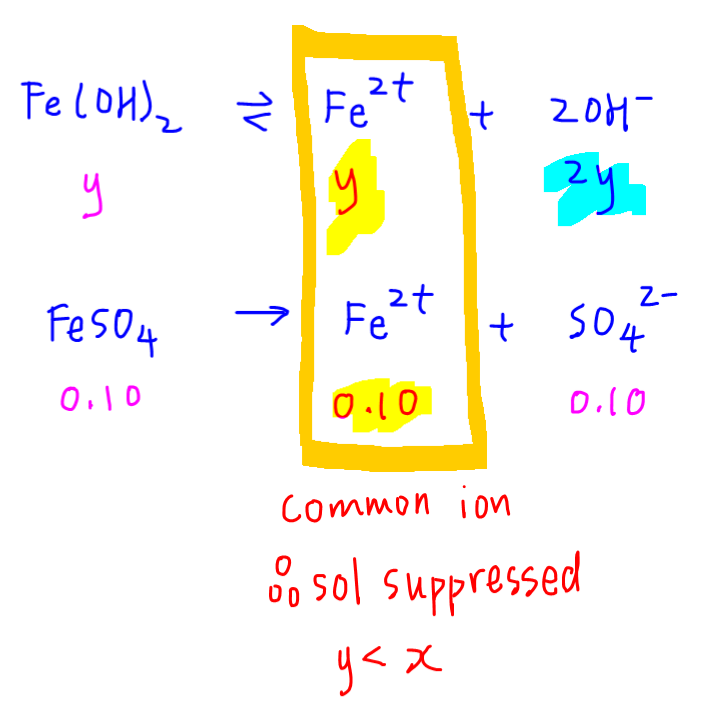
Let y be solubility of iron hydroxide, it will dissociate to give y moldm-3 Fe2+ and 2y moldm-3 OH-.
0.10 moldm-3 of FeSO4 will dissociate to give 0.10 moldm-3 of Fe2+ and SO42-.
Notice both salts give Fe2+, so Fe2+ is the common ion.
Based on the common ion effect, we know that solubility of Fe(OH)2 will be suppressed, so we expect solubility in FeSO4 (y) to be lower than solubility in water (x).
Check out this video lesson to learn more about the common ion effect.
Writing the Ksp expression for Fe(OH)2:
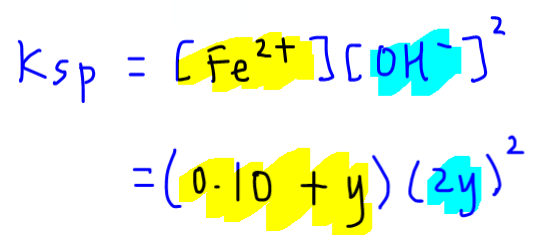
Concentration of Fe2+ in the system will include contribution of Fe2+ from both salts = (0.10 + y)
Concentration of OH- = 2y
We need to approximate and simplify the calculation as solving for quadratic equation is not in A Level Chemistry syllabus.

We assume contribution of Fe2+ from the sparingly soluble salt to be negligible, hence conc of Fe2+ will be 0.10 moldm-3.
We can now calculate solubility (y):
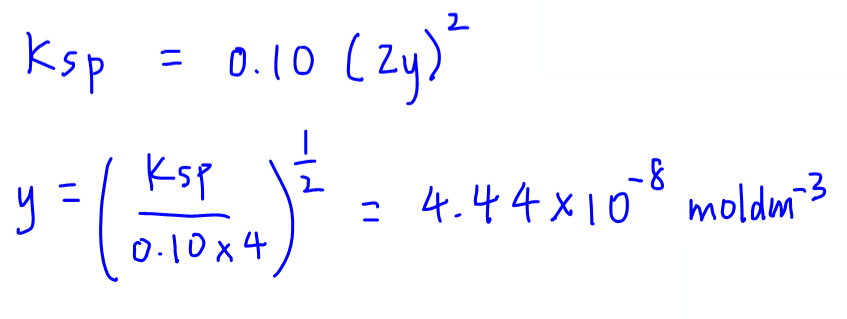
This is a smaller value as compared to solubility of Fe(OH)2 in water (previously calculated to be 5.82 x 10-6 moldm-3) which is consistent with the common ion effect.
Topic: Solubility Product, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
