Combustion Analysis of H2S and CS2
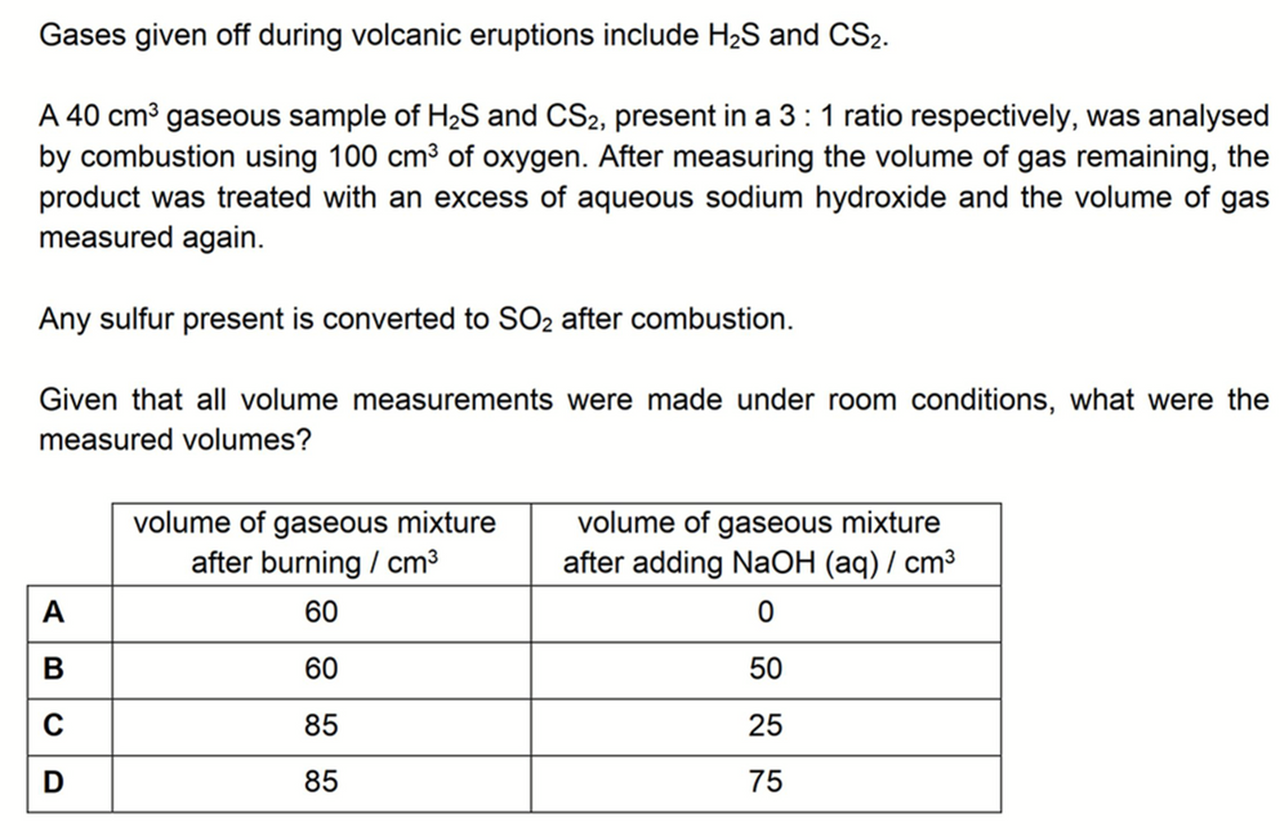
H2S and CS2 are present in 3 to 1 ratio, hence volumes of H2S and CS2 are 30 cm3 and 10 cm3 respectively.
Question mentioned any sulfur present is converted to SO2 after combustion, and we will expect hydrogen to be converted to H2O and carbon converted to CO2 after combustion.
Then we can balanced the equations for combustion of H2S and CS2.
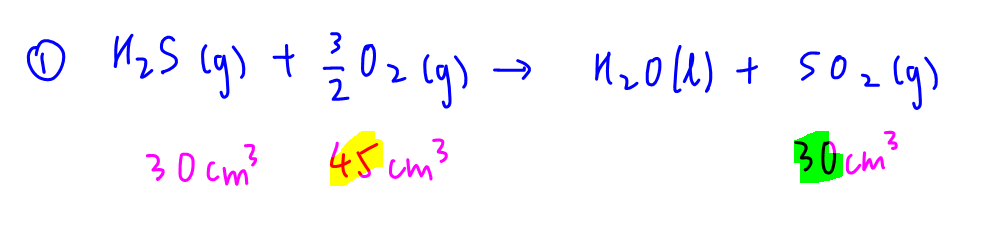
Comparing mole ratio of gases we can deduce 30 cm3 of H2S reacts with 45 cm3 of O2 to form 30 cm3 of SO2.
We can ignore water produced since it is liquid at room temperature and does not contribute to volume of gases.
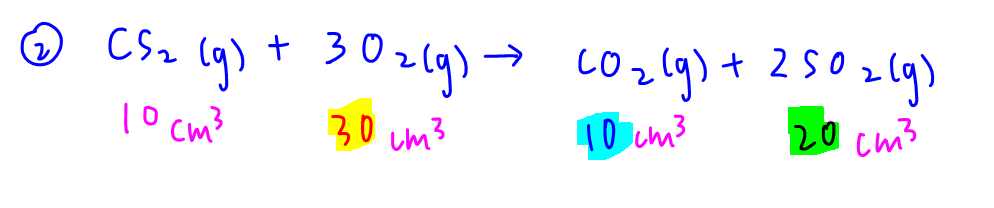
10 cm3 CS2 reacts with 30 cm3 O2 to form 10 cm3 CO2 and 20 cm3 SO2.
Now we can put everything together:
Total volume of O2 reacted = 45 + 30 = 75 cm3
Vol of O2 remaining = 100 - 75 = 25 cm3
Vol of CO2 formed = 10 cm3
Vol of SO2 formed = 30 + 20 = 50 cm3
Hence,
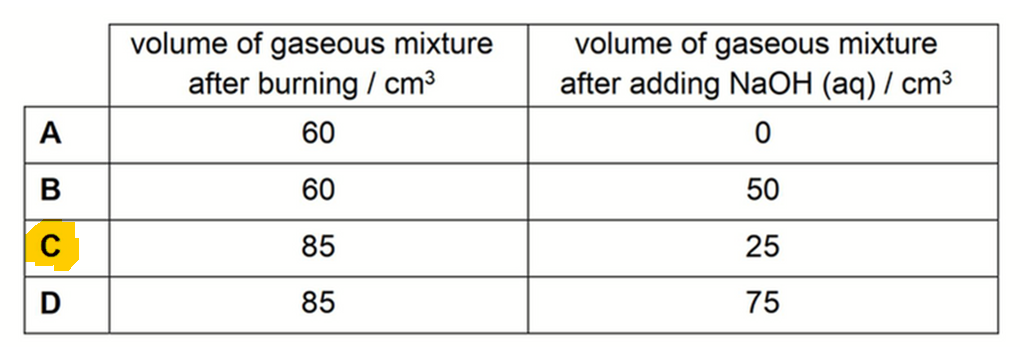
Vol of gases after burning = vol of O2 remaining + vol of CO2 + vol of SO2 = 25 + 10 + 50 = 85 cm3
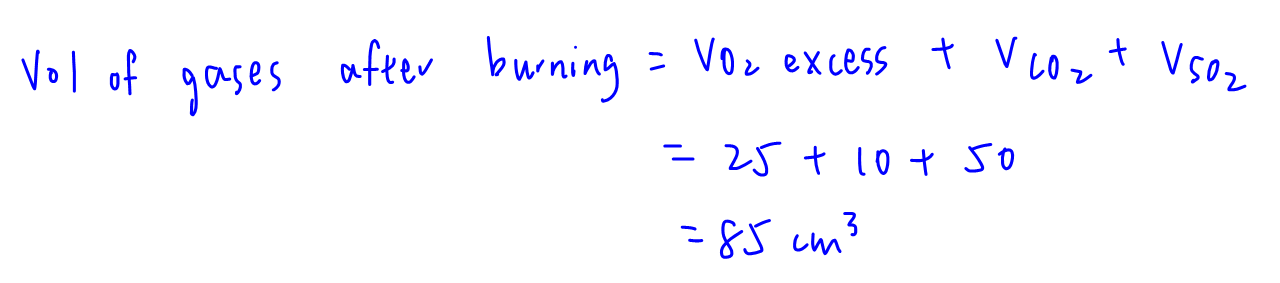
After treated with excess NaOH(aq), acidic gases CO2 and SO2 will be absorbed.
Final vol after adding NaOH(aq) = 25 cm3
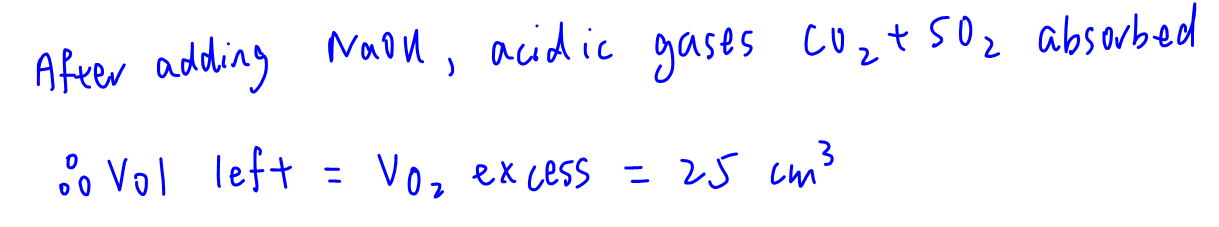
Therefore answer to this question is option C.
Topic: Mole Concept, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
