Compare Acidity of Organic Compounds - Worked Example
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through this week's question.
We are required to sort the following compounds in increasing pH or decreasing acid strength:
chloroethanoic acid, ethanoic acid, ethanoyl chloride and ethyl ethanoate
In this list we have 2 carboxylic acids, 1 acid chloride and 1 ester.
Let's consider the acidity of each functional group.
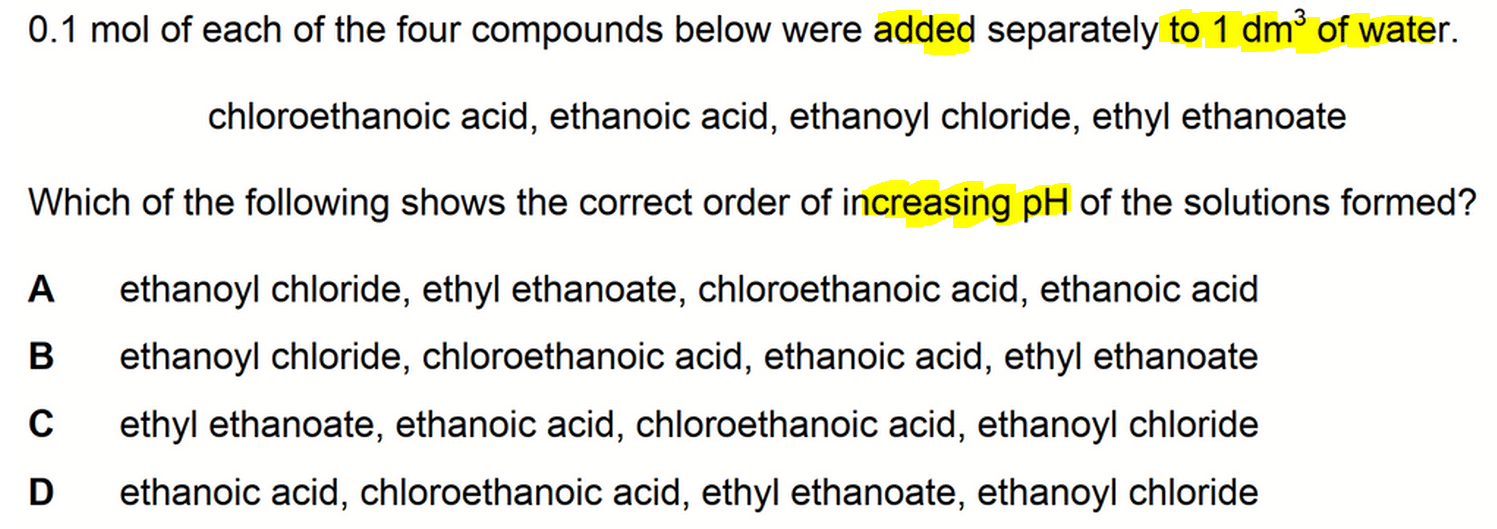
1. Aqueous solution of Acid Chloride
Anhydrous acid chloride is neutral since there are no H+ to donate.
In aqueous medium however, acid chloride can react with water to form carboxylic acid and HCl.
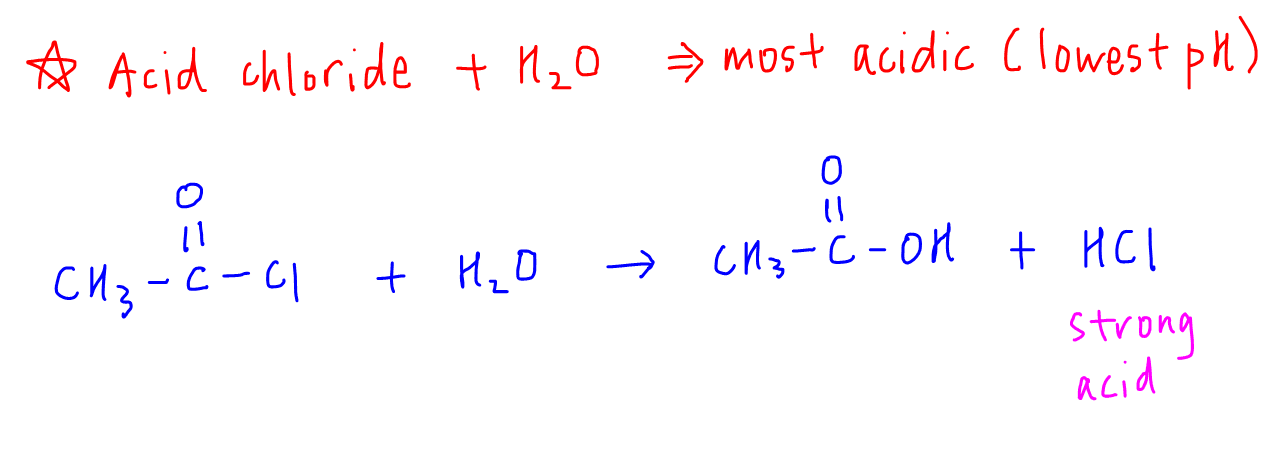
Since HCl is a strong acid, it will dissociate fully to give a high concentration of H+(aq).
Hence an aqueous solution of ethanoyl chloride will be the most acidic.
2. Ester
Esters have no protons to donate hence they are neutral.
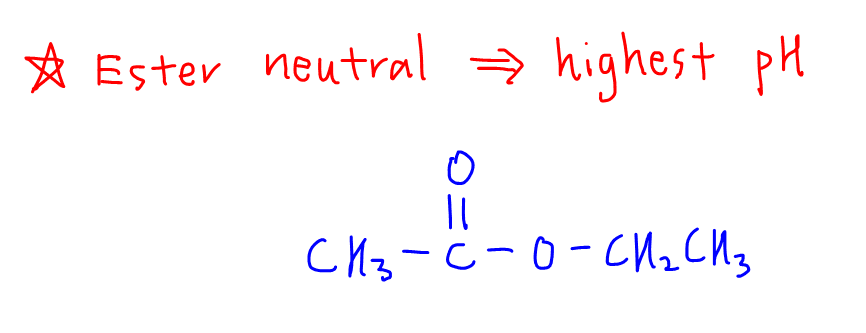
For this question we will expect ethyl ethanoate to have the highest pH.
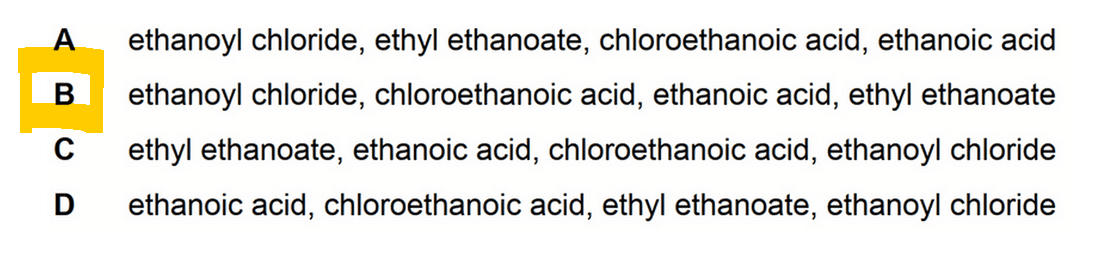
Hence the answer to this question will be option B.
3. Comparing Acids
Let's compare the acidity of chloroethanoic acid and ethanoic acid.
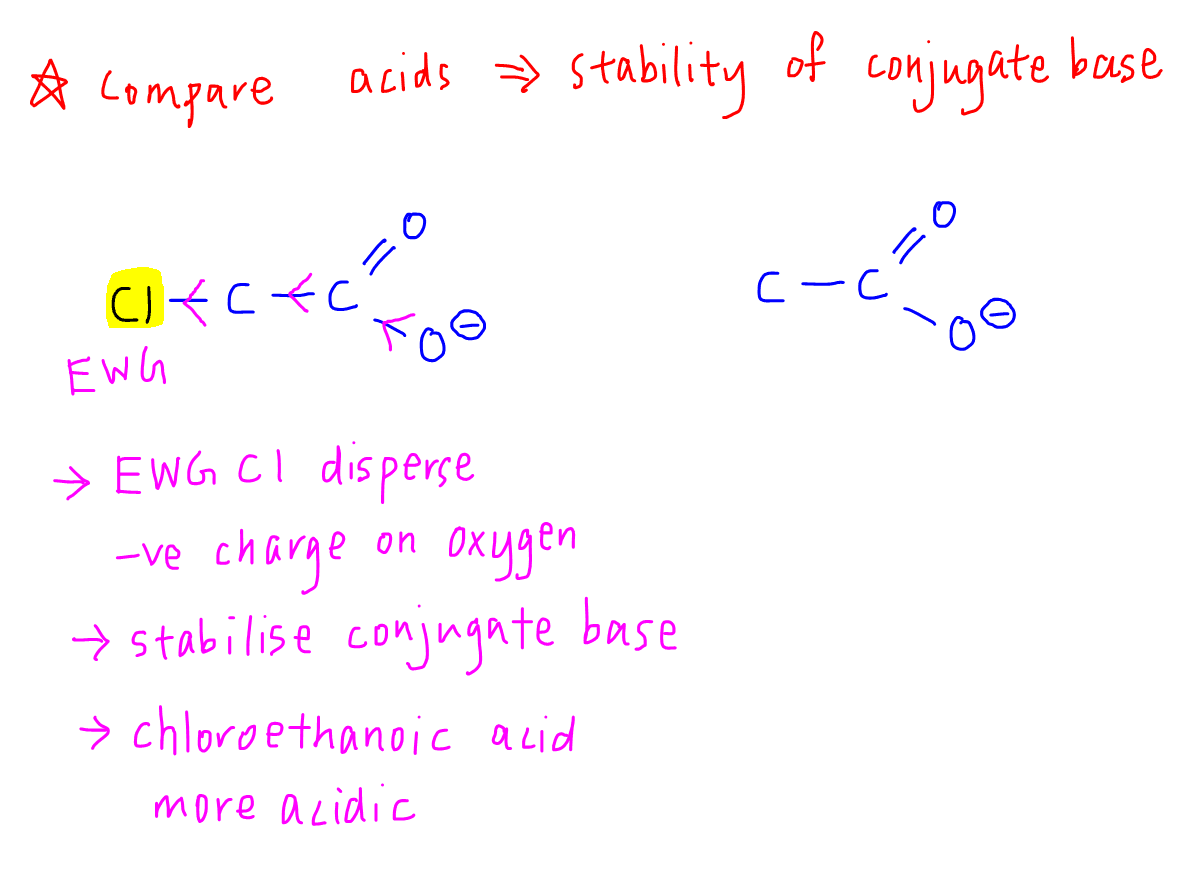
For chloroethanoic acid, the electron withdrawing Cl group will disperse negative charge on O- of conjugate base.
The conjugate base will be more stable, hence chloroethanoic acid will be more acidic than ethanoic acid.
For comparing acidity of carboxylic acids, phenols and alcohols, check out the video that I've done previously.
Topic: Carboxylic Acids and Derivatives, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
