Compare Bond Angle of Species

To compare bond angles we need to
- determine the number of bond pair and lone pair around central atom via dot-cross diagram
- deduce its shape using VSEPR concept
Learn how to draw dot-cross diagram.
Learn how to use VSEPR to deduce shapes of molecules.
A. ICl3

Central atom (I) has 3 bond pairs and 2 lone pairs.
Shape is T-shape, bond angle 86 degrees.
B. SF3+
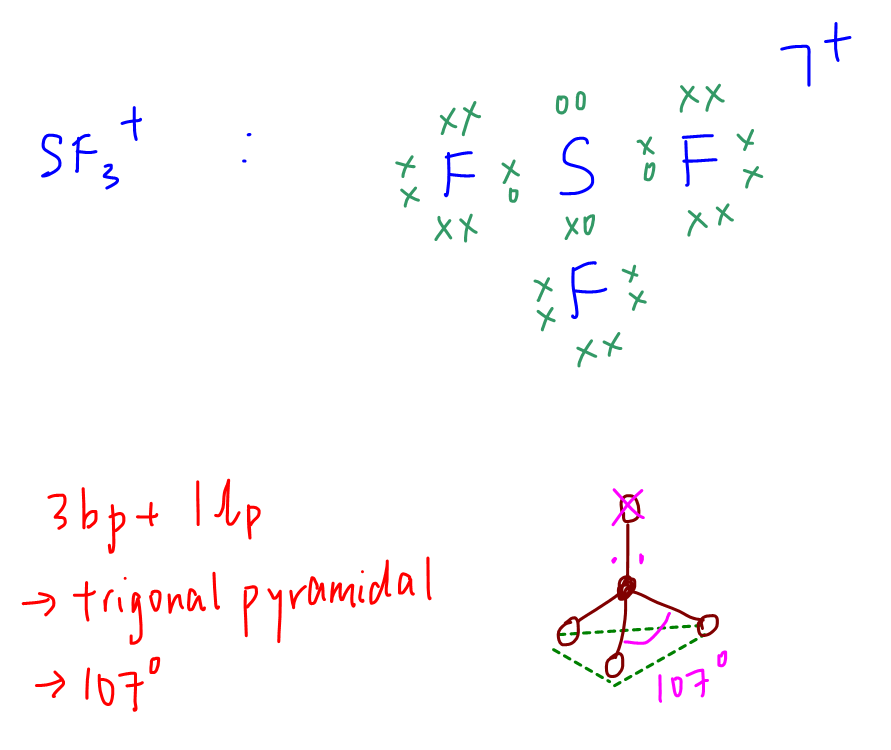
Central atom (S) has 3 bond pairs and 1 lone pair.
Shape is trigonal pyramidal, bond angle 107 degrees.
C. ClO3-
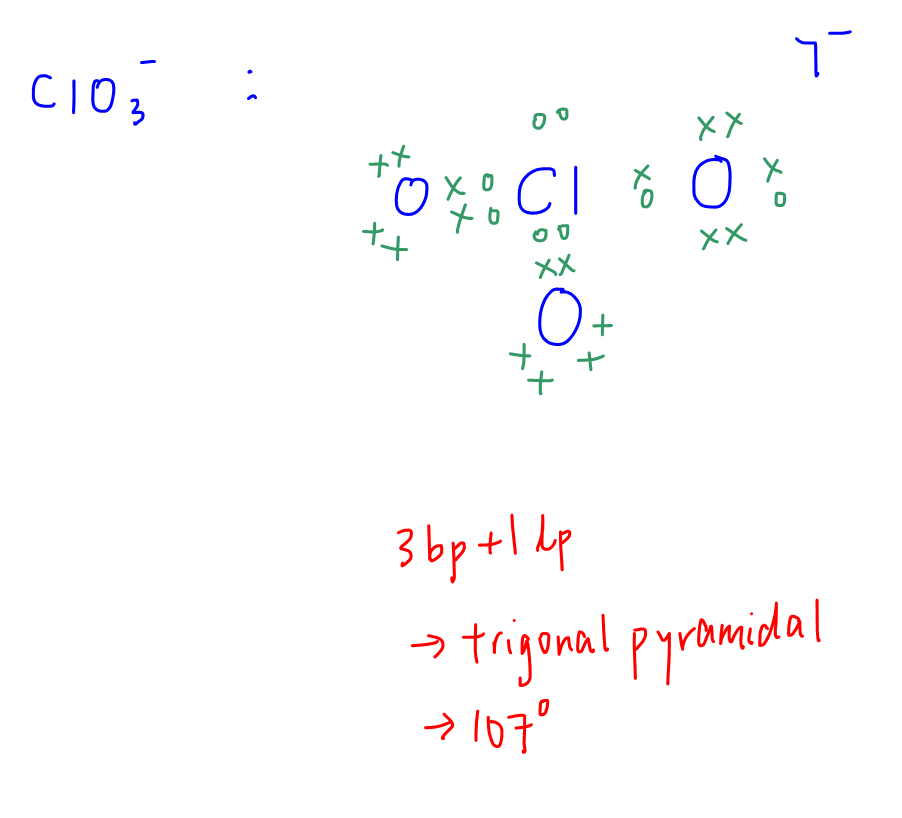
Central atom (Cl) has 3 bond pairs and 1 lone pair.
Shape is trigonal pyramidal, bond angle 107 degrees.
D. N2H4
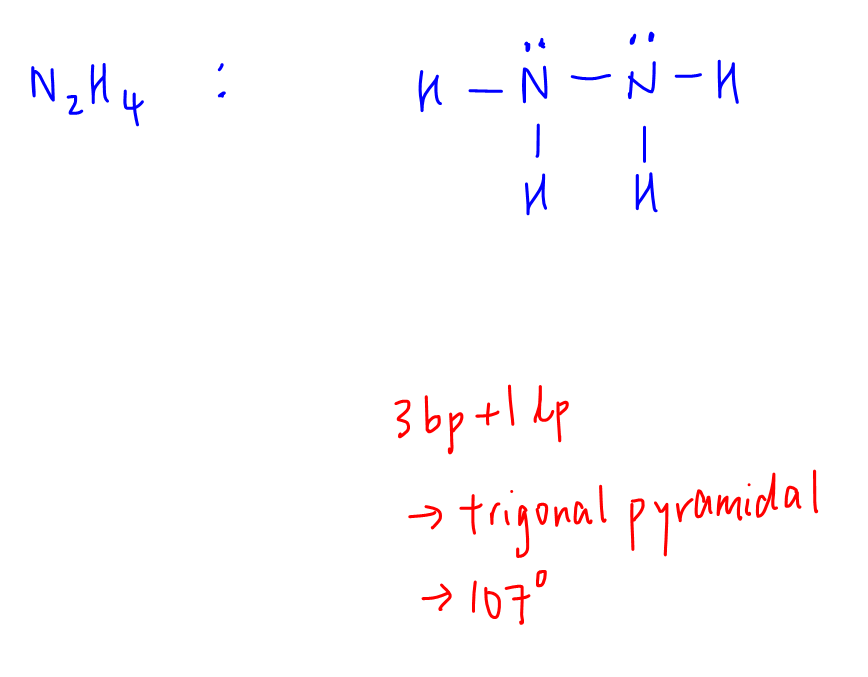
Central atom (N) has 3 bond pairs and 1 lone pair.
Shape is trigonal pyramidal, bond angle 107 degrees.
Therefore the answer to this question is option A.
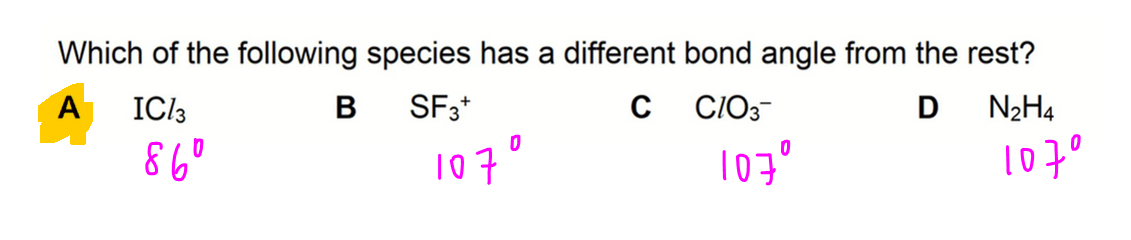
Topic: Chemical Bonding, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
