Compare Melting Point of Ionic Compounds
In this video lesson created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre let us compare the melting point of ionic compounds.
The strength of ionic bonds in ionic compounds is related to lattice energy via the following expression:

where
q+ is the charge of cation
q- is the charge of anion
r+ is the radius of cation
r- is the radius of anion
Notice the denominator term (r+ + r-) is simply the distance between the cation and anion.
So in general, the greater the charges of the ions and the shorter the distance between the ions, the magnitude of lattice energy will be greater.
This means more energy is required to overcome the stronger ionic bonds and that ionic compound will have a higher melting point.
In most questions, we only need to consider one of the 4 terms in the lattice energy expression.
Let's compare the melting points between Na2O and MgO as an illustration.
The difference will be in the cations Na+ and Mg2+.
Mg2+ has a higher charge and smaller radius than Na+, but the 2x increase in charge is more significant than the small difference in radius so we can just focus on the q+ term.
The difference in radius is small because both Na+ and Mg2+ are isoelectronic with 10 electrons, so they will both have 2 filled principal quantum shells with roughly the same size.
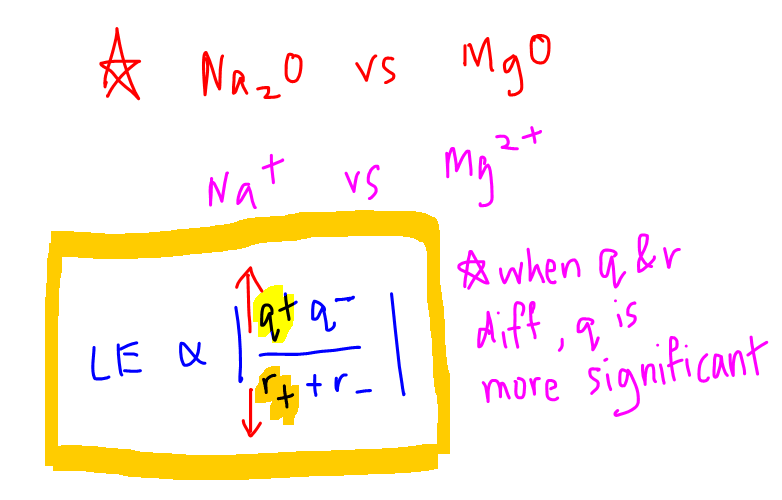
The lattice energy of MgO will be greater, ionic bonds in MgO are stronger and hence MgO will have a higher melting point than Na2O.
Topic: Chemical Bonding, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
