How to Compare Melting Point of Metals
Let us explain the melting point trend of the following metals:
Na - 97.7oC Mg - 650oC Al - 660oC
We notice there is an increase in melting point across these Period 3 metals.
During melting, energy is required to overcome strong metallic bonds between metal cations and the sea of delocalised electrons.
There are 2 factors affecting the strength of metallic bonds and melting point of metals.
1. Valency

The greater the valency of the metal, more valence electrons can be delocalised to form more positively charged cations and a bigger sea of delocalised electrons.
Comparing sodium and aluminium:
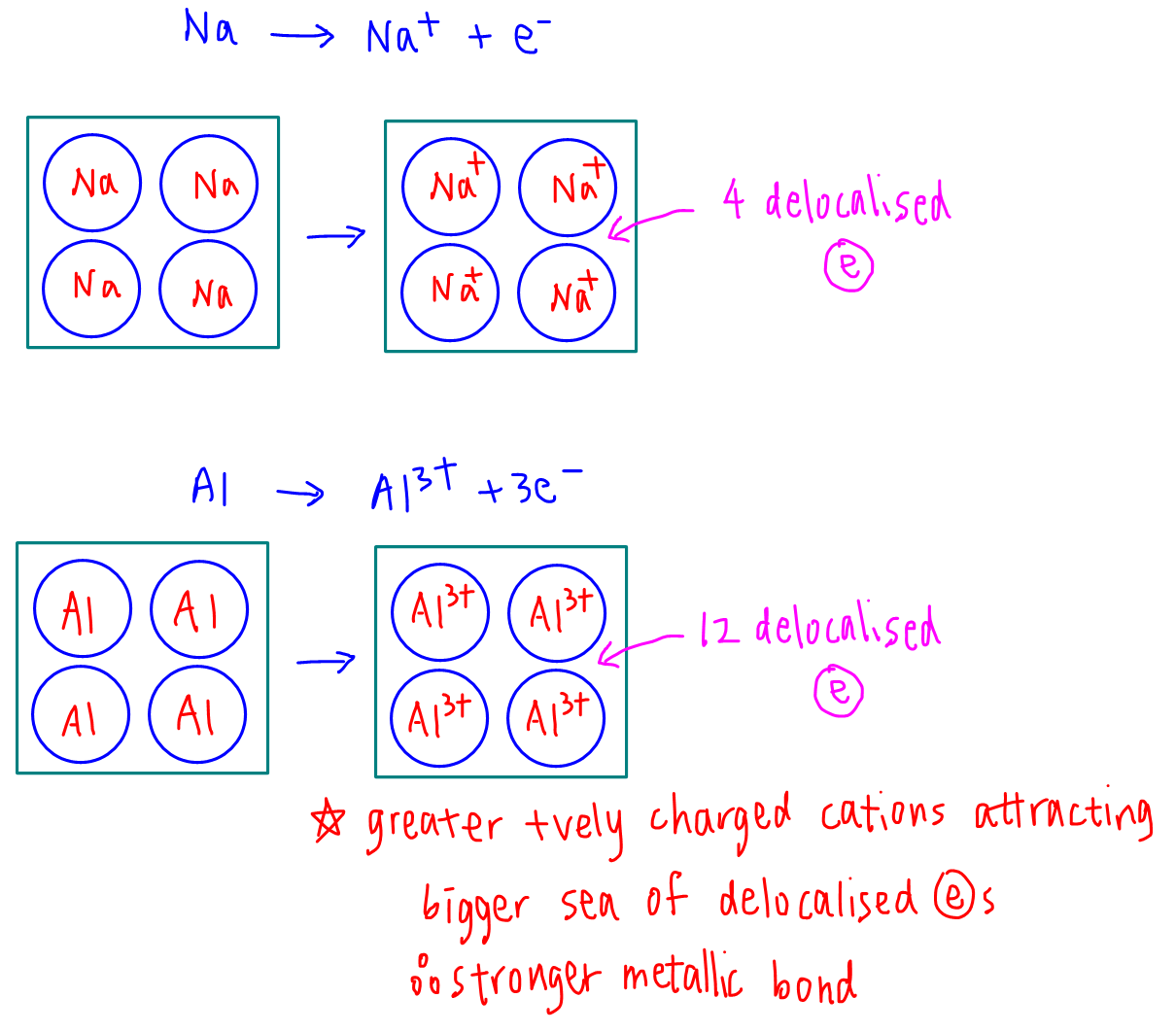
Aluminium forms more positively charged Al3+ with a bigger sea of delocalised electrons, hence metallic bonds in aluminium is stronger and more energy is required to overcome it.
Therefore melting point of metals increase across Period 3 from sodium to aluminium.
2. Charge Density

Since metals down a Group have the same valency, we have to look at charge density instead to compare strength of metallic bonds.
Down the group, size of metal cations increase due to increase in number of filled principal quantum shells.
Charge density of metal cation which is proportionate to charge/size will decrease.
The metal cation will have a weaker attraction on the delocalised electrons hence metallic bonds are weaker.
Therefore melting point decreases down the Group for metals.
Topic: Chemical Bonding, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
