Compare Nucleon, Proton and Neutron Numbers - 2019 P1 Q1
Hi folks, Happy 2020!
In this first video of the year, let's check out Question 1 of 2019 A Level H2 Chemistry Paper 1:
We know that:
- proton number or atomic number is the number of protons in the nucleus
- neutron number is the number of neutrons in the nucleus
- nucleon number or mass number is the number of protons and neutrons in the nucleus
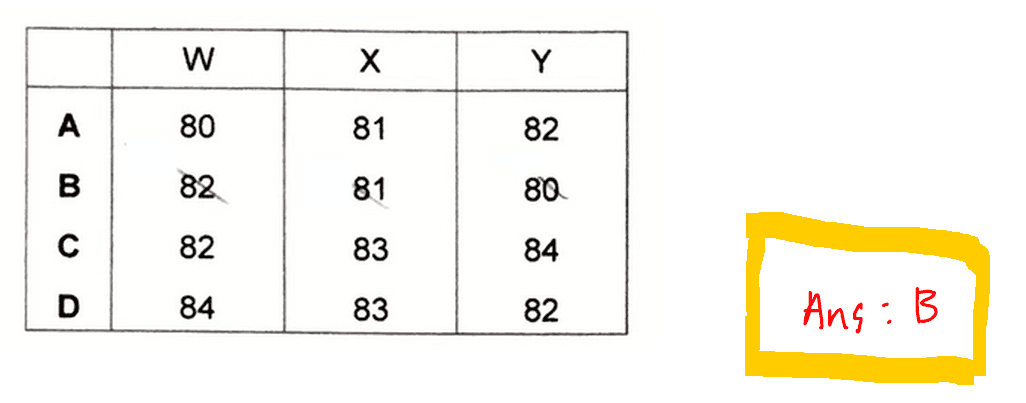
So we need to use the information given in the question to deduce the proton number and neutron number of unknown species W, X and Y.
1. Deduce Proton Number
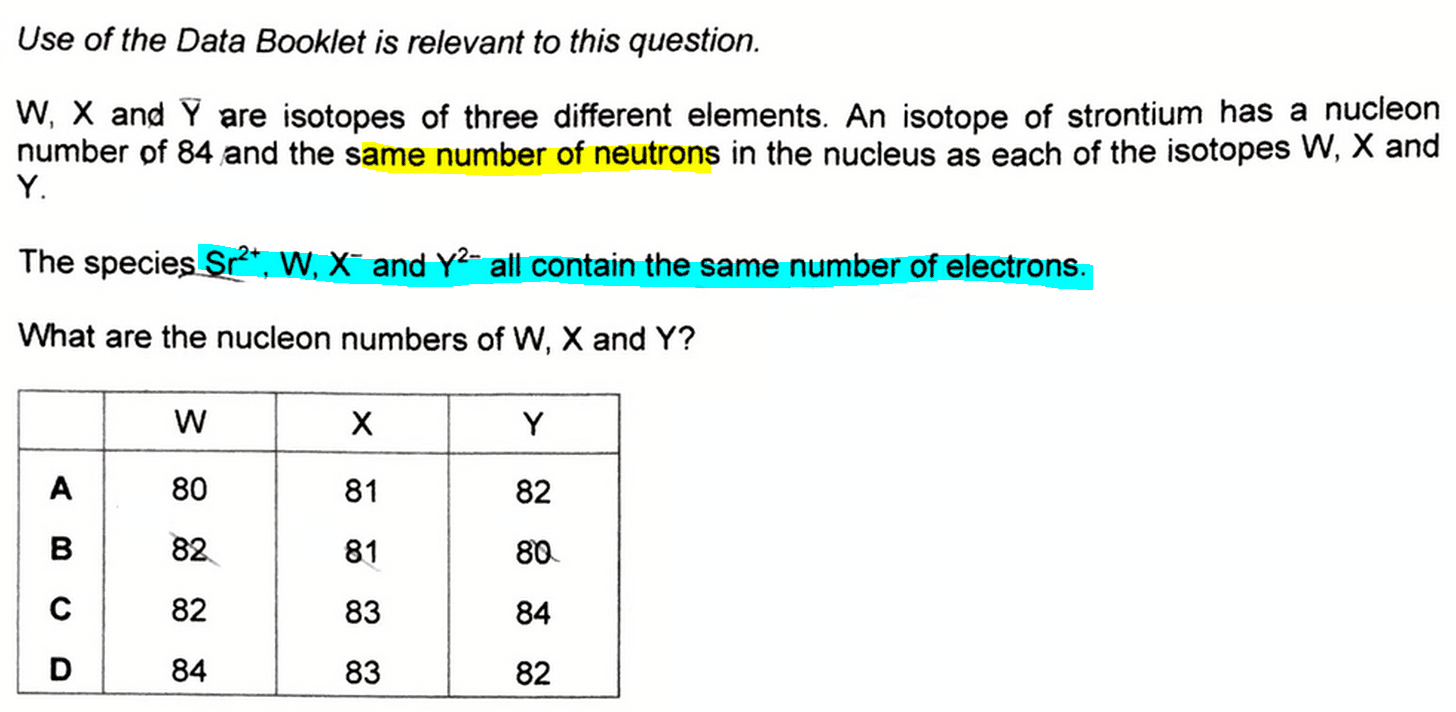
We are given that the species Sr2+, W, X- and Y2- have the same number of electrons.
So from known element strontium which has a proton number of 38, we can deduce Sr2+ has 36 electrons.
This means W, X- and Y2- each has 36 electrons, since they are isoelectronic.
We can then deduce the proton number of each of these species: W-36, X-35 and Y-34.
2. Deduce Neutron Number
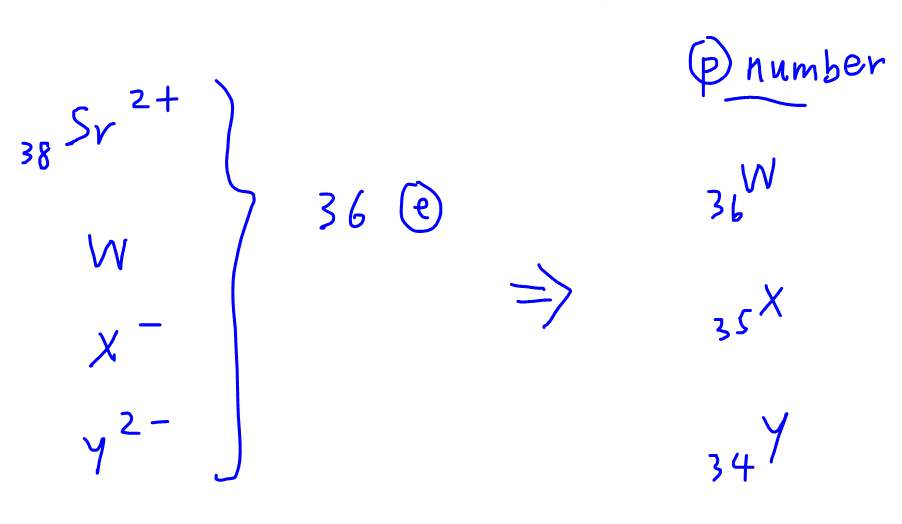
We are also given that the isotope of Sr with mass number 84 has the same number of neutrons as the unknown species.
So we can work out the neutron number for Sr which is 46.
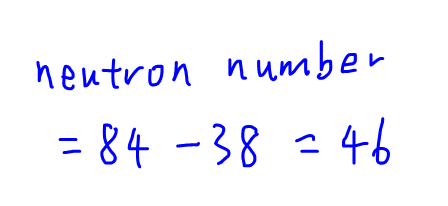
Hence W, X and Y each has 46 neutrons.
3. Deduce Nucleon Number
Finally we can determine the nucleon number for the species which is just adding up the proton and neutron numbers together.
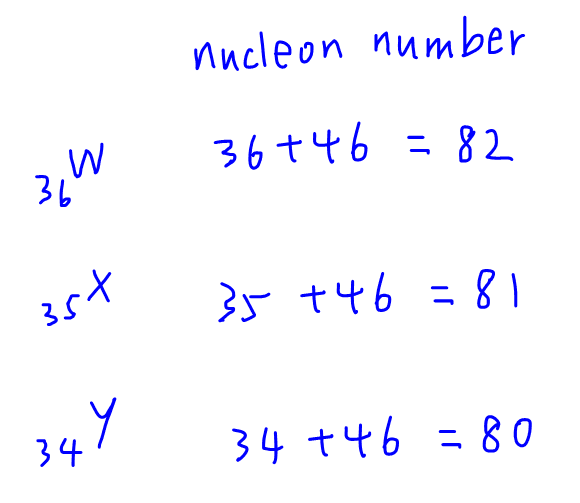
Finally we can compare the options and determine the answer to this question is B.
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
