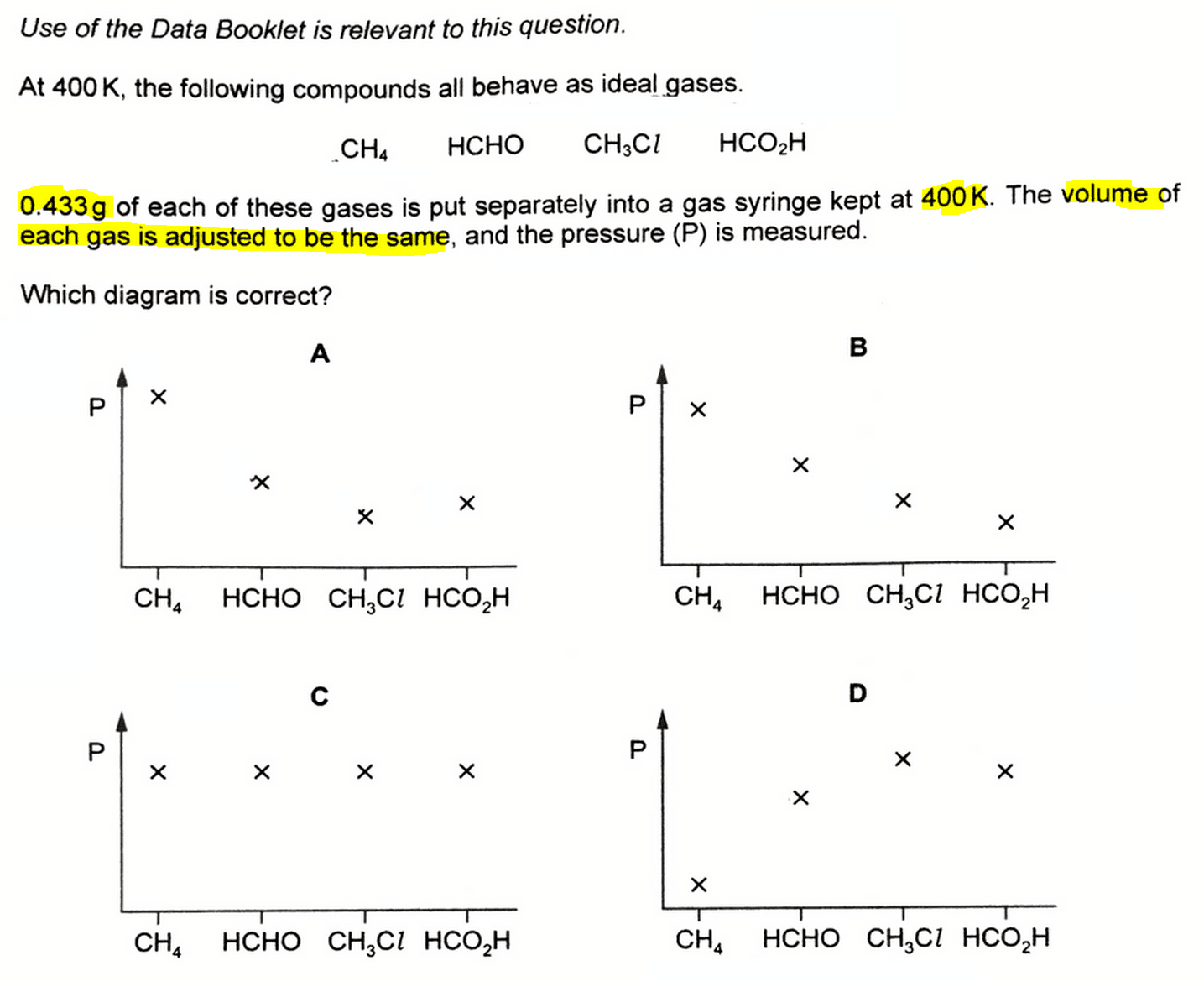
2019 P1 Q6 - Compare Pressure of Gaseous Compounds
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to discuss 2019 A Level H2 Chemistry Paper 1 Question 6.
To determine the relationship between pressure and gaseous compounds of various molar masses, we need to use the Ideal Gas Equation.
From the ideal gas equation PV = nRT, we can write moles(n) as mass(m) / molar mass(M).
Given mass, temperature(T) and volume(V) are all constant, we can combine these terms together with Gas Constant(R) to form a big constant term.
This will give us the relationship between pressure(P) and molar mass.
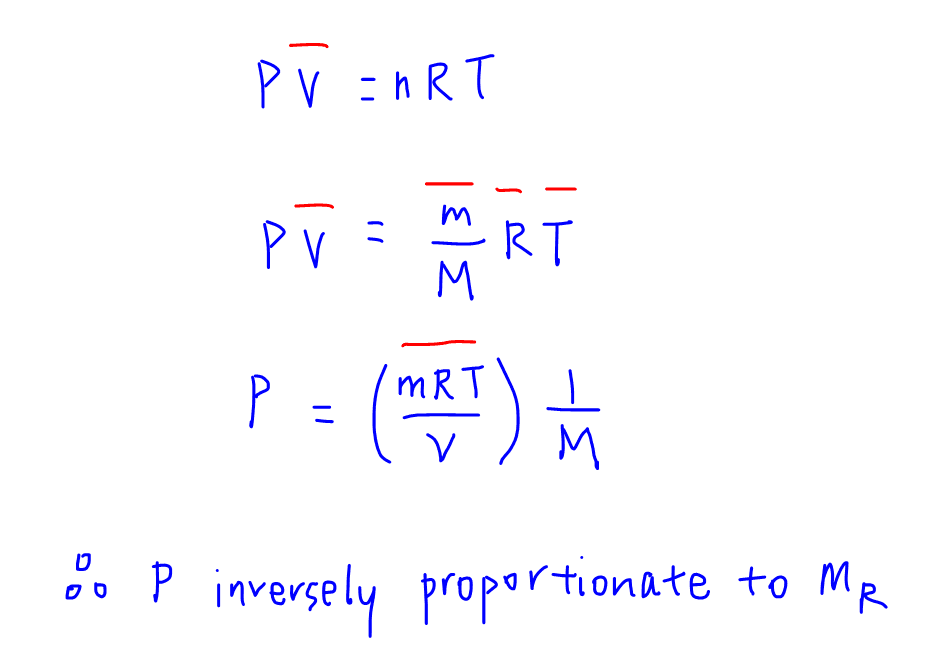
Hence we can deduce pressure is inversely proportionate to molar mass.
This means the smaller the molar mass of the compound, the higher the pressure.
We can now look back at the list of compounds given to work out their molar masses.

CH4 with the smallest molar mass will have the highest pressure, while CH3Cl with the biggest molar mass will have the lowest pressure.
Finally we can look back at the options and choose the graph where CH4 has the highest pressure and CH3Cl has the lowest pressure.
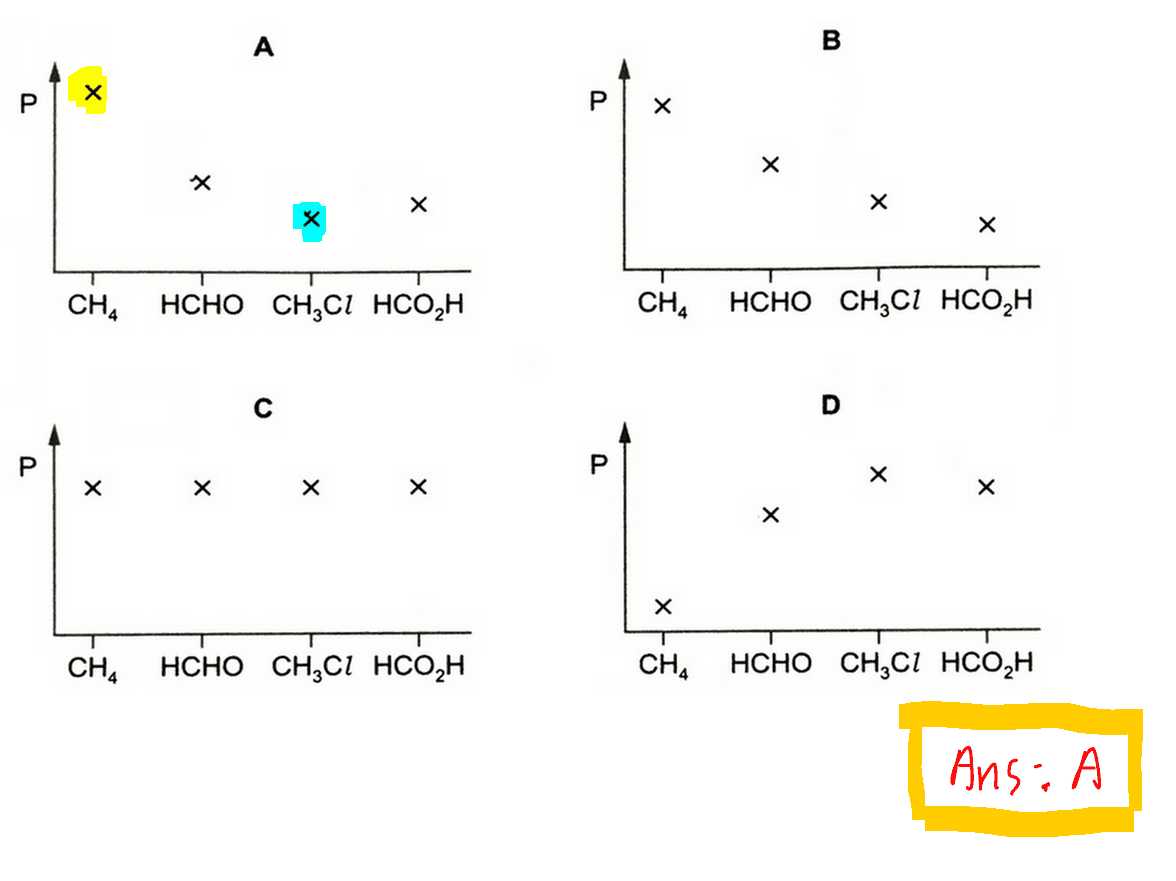
Hence the answer to this question is A.
Topic: Gaseous State, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
