Compare Reactivity of Alcohol and Phenol
In phenol -OH group is directly bonded to benzene.
The lone pair of oxygen can interact with the delocalised pi system of benzene and resonance stability is extended to C-O bond.
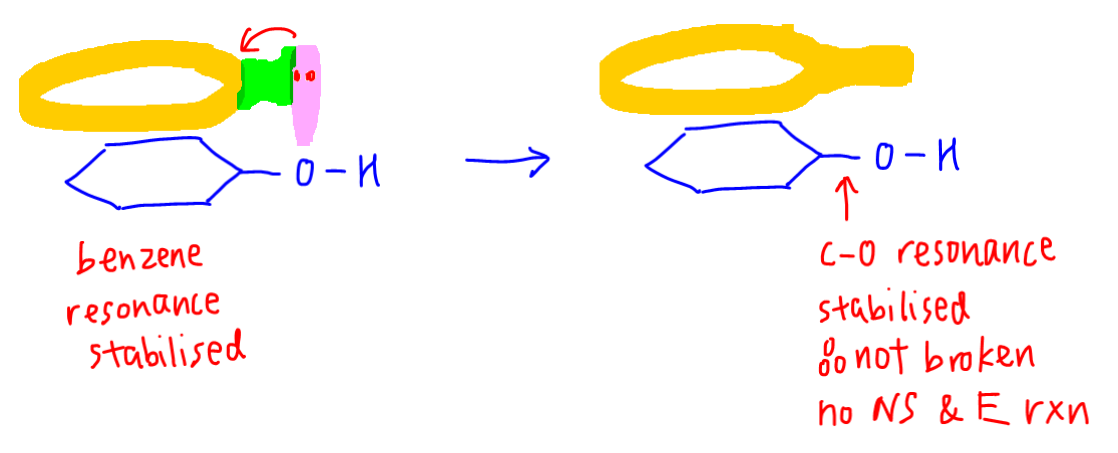
This makes the C-O bond very stable and phenol does not take part in substitution and elimination reactions like alcohol.
Therefore phenol will have significantly fewer reactions compared to alcohol, since both alcohol's C-O and O-H bonds can be broken.
Let's compare the reactivity and types of reaction for alcohol and phenol.
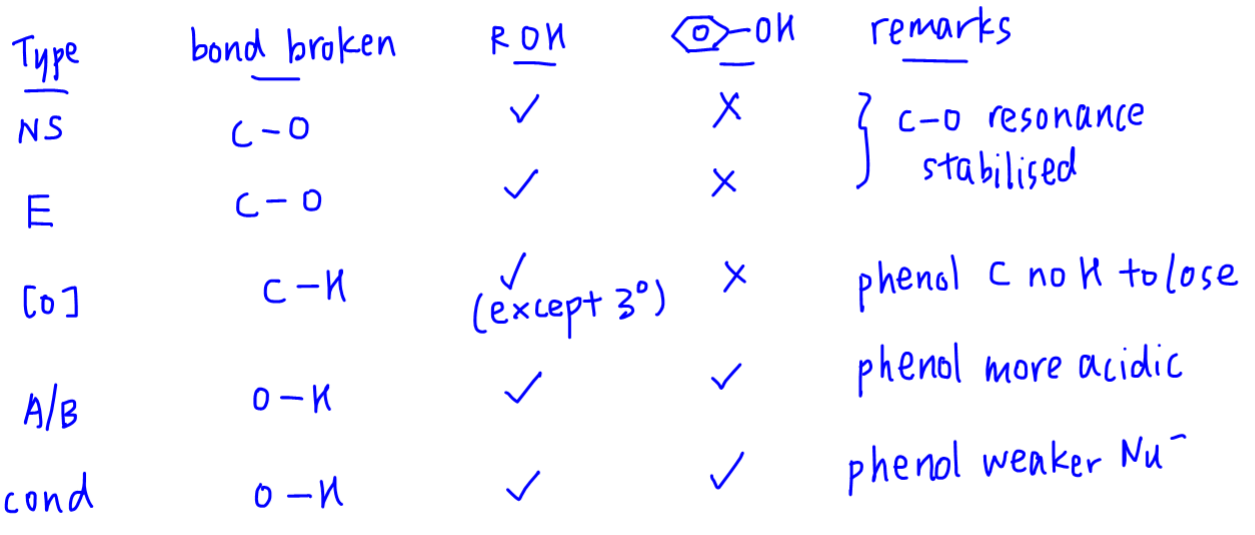
For alcohols:
Types of reaction when C-O bond is broken include nucleophilic substitution and elimination.
Types of reaction when O-H bond is broken include oxidation (C-H bond is also broken), neutralisation and condensation.
For phenols:
Since C-O bond is resonance stabilised and not broken, there is no nucleophilic substitution and elimination.
O-H bond can still be broken hence phenols can take part in neutralisation and condensation reactions.
Check out this video to learn all the reactions of alcohols and phenols.
Topic: Alcohol, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
