Comparing Acidity of Organic Compounds
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to compare acidity of some organic compounds that we learn in A Level Chemistry.
Let's consider the dissociation of a weak acid HA to form H+ and conjugate base A-.
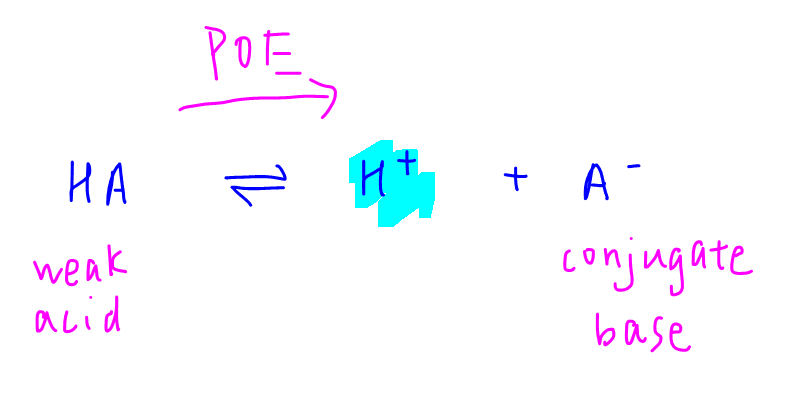
If the conjugate base is more stable, the system will favour the formation of it by shifting the position of equilibrium towards the right.
This releases more H+ hence it makes weak acid HA more acidic.
Therefore when we want to compare acidity in organic chemistry, we focus on the stability of conjugate base - the more stable the conjugate base, the stronger the acid.
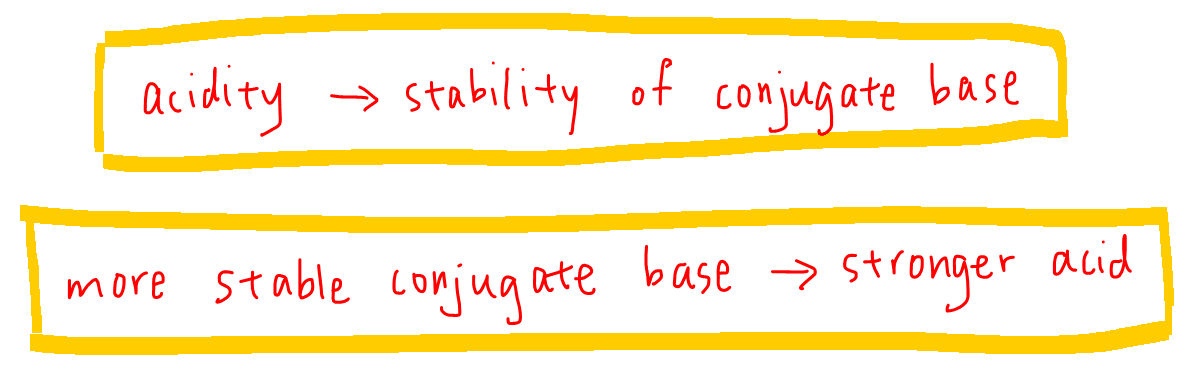
Comparing Organic Compounds
Here is the acidity trend of functional groups.
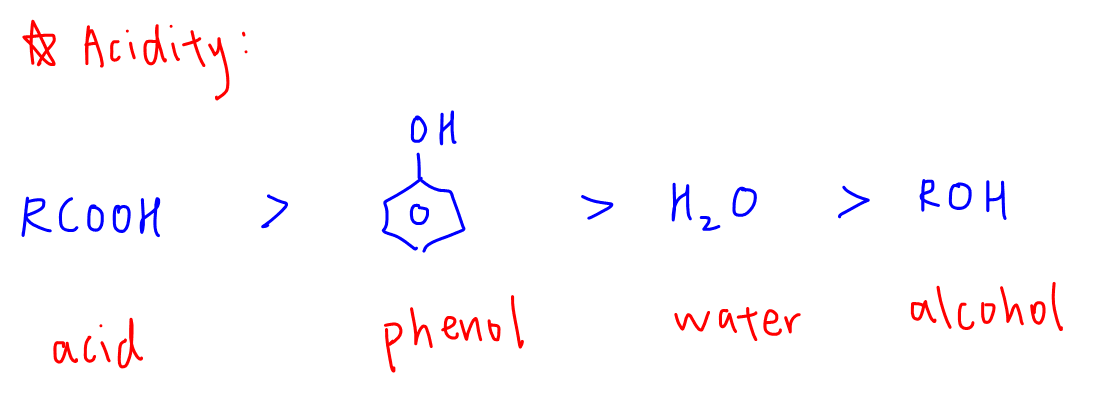
For each of the functional groups, we can look at the conjugate base and deduce its stability to explain the acidity of each compound.
Carboxylic Acid
Acids will dissociate to give conjugate base carboxylate and H+.
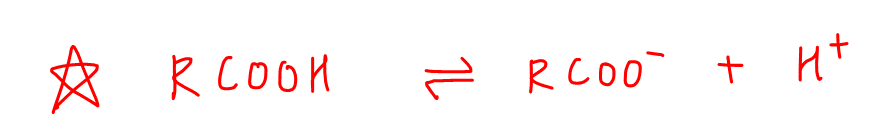
The carboxylate is resonance stabilised to a very significant extent, as the negative charge on oxygen is delocalised extensively between 2 electronegative oxygens.
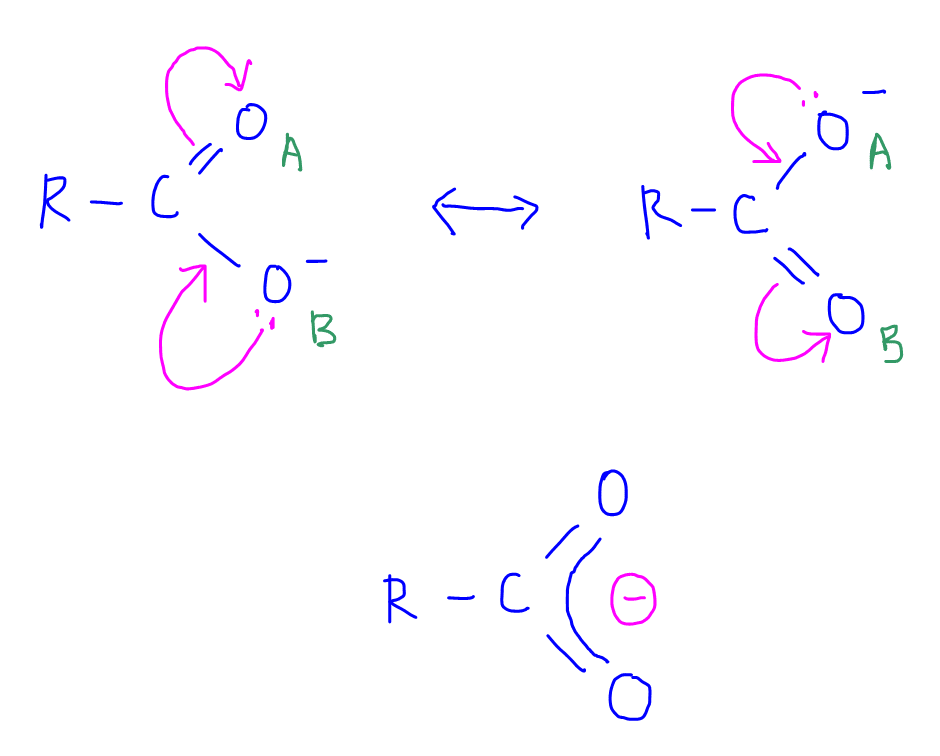
This makes the carboxylate very stable, hence carboxylic acid is the most acidic functional group.
Phenol
Phenols will dissociate to give conjugate base phenoxide and H+.
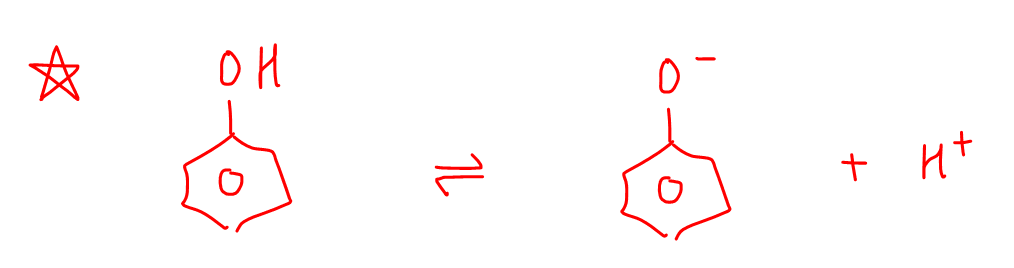
The phenoxide is also resonance stabilised as the negative charge on oxygen can be delocalised into the pi electron system of benzene.
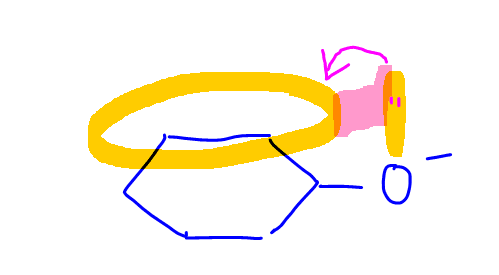
This makes phenoxide more stable hence phenol is more acidic than water.
Water
Even though water is not an organic compound, putting water into the comparison is useful.
Water will dissociate to give hydroxide and H+.
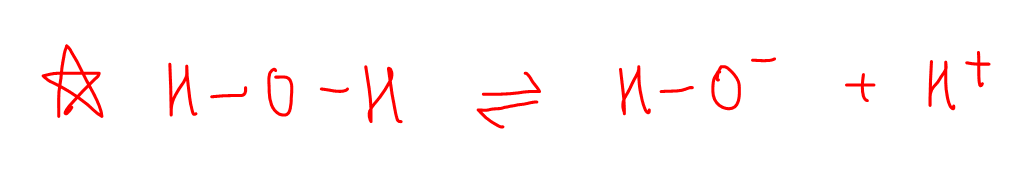
Hydrogen attached to oxygen atom is neither electron donating nor electron withdrawing.
Thus it does not have any destabilising or stabilising effect on the negative charge on oxygen.
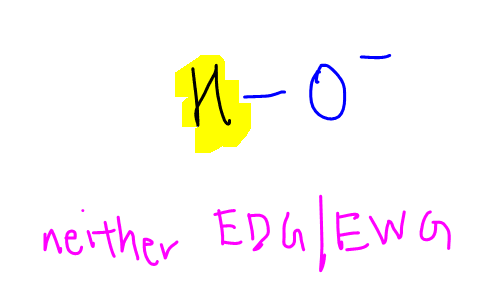
This makes water neutral and a useful benchmark to compare acidity of other organic compounds.
Alcohol
Finally, alcohols will dissociate to give alkoxides and H+.
The oxygen atom is attached to electron donating alkyl group which intensifies the negative charge on oxygen.
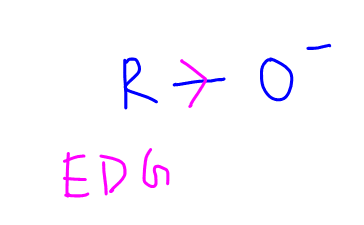
This destabilises the alkoxide conjugate base and hence alcohol is less acidic than water.
For the detailed step-by-step discussion on how to compare acidity of organic compounds, check out this video!
Topic: Carboxylic Acids and Derivatives, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
