Comparing Bond Energy Using Hybridisation
Let's start our discussion with these 2 compounds, methane and ethene.
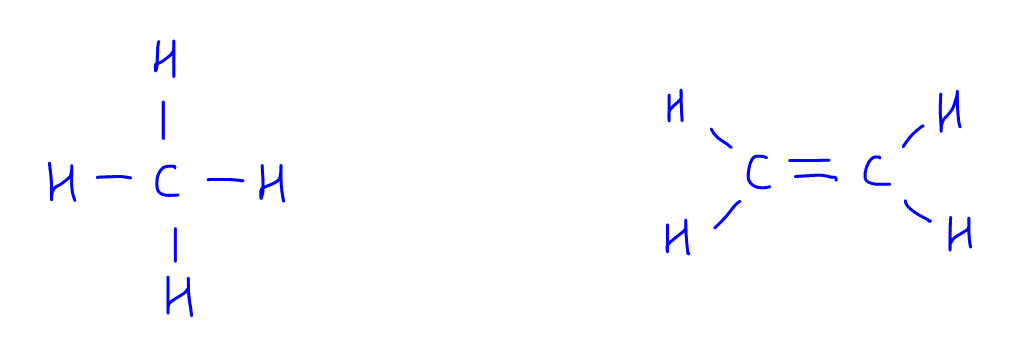
If we want to compare the C-H bond energy in both compounds, which has a more stable C-H bond?
You notice we cannot use bond energy values from the Data Booklet to figure this out, as there is only 1 C-H bond energy value in the Data Booklet!
So therefore we would need to look at the state of hybridisation of carbon to eventually compare the C-H bonds in both compounds.
Hybridisation
Hybridisation is the mixing of valence orbitals to form sigma bonds.
I have a previous detailed video lesson on hybridisation of carbon so if you are not sure about this concept, do give this video a look!
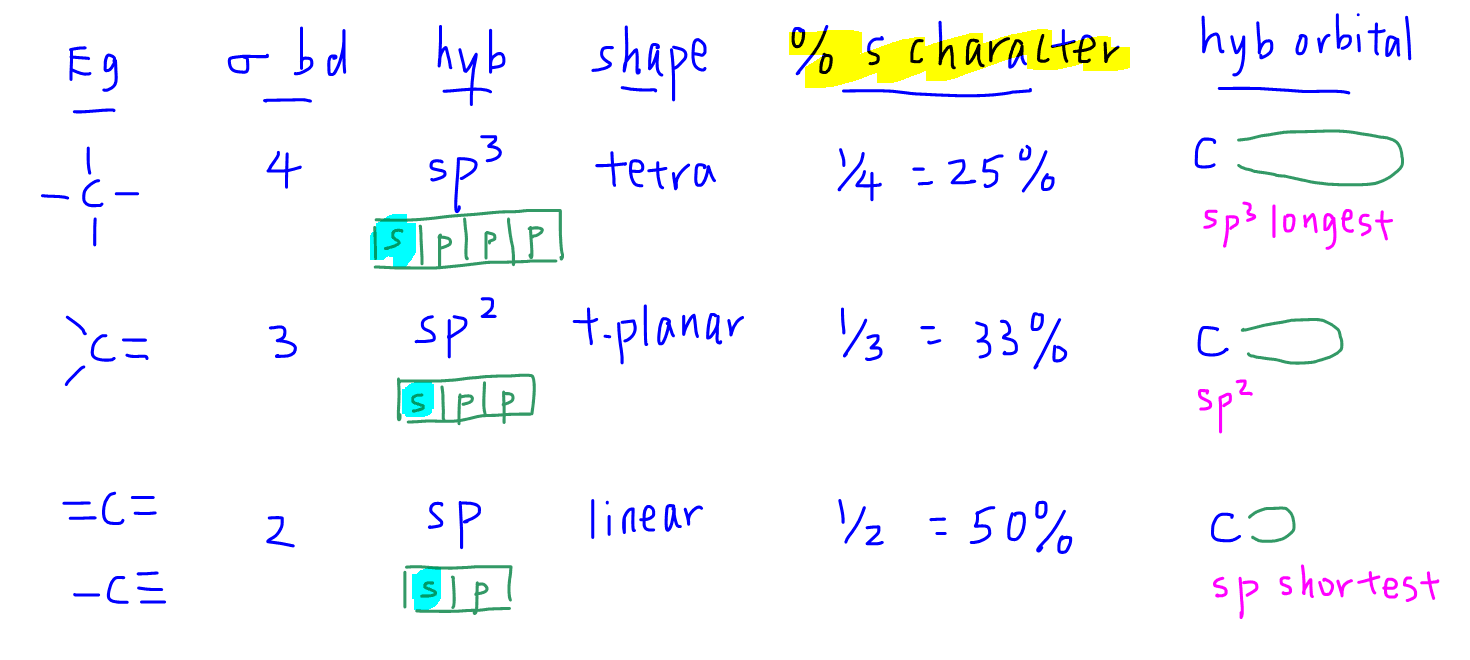
The table above summarises the different states of hybridisation of carbon, the percentage s character of the hybridised orbitals, and the length of orbitals.
- sp3 hybridised orbital has 25% s character and is longer and further away from the nucleus
- sp hybridised orbital has 50% s character and is shorter and closer to the nucleus
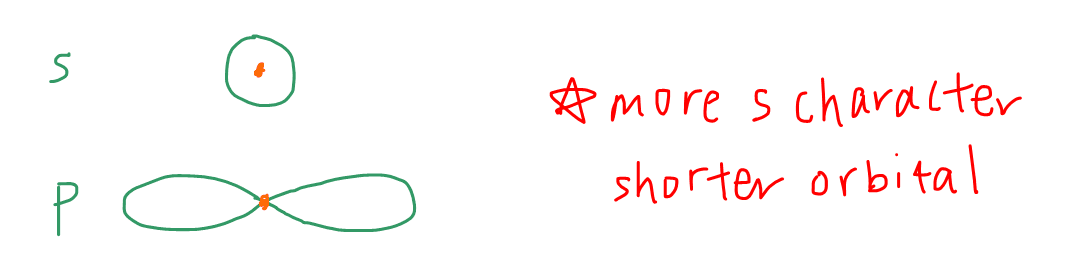
The reason why a hybridised orbital with greater s character is shorter is because s orbital is more stable and closer to the nucleus than p orbital.
1. Compare C-H Bond
So back to our example of methane and ethene, we can first determine the state of hybridisation of the carbons first.
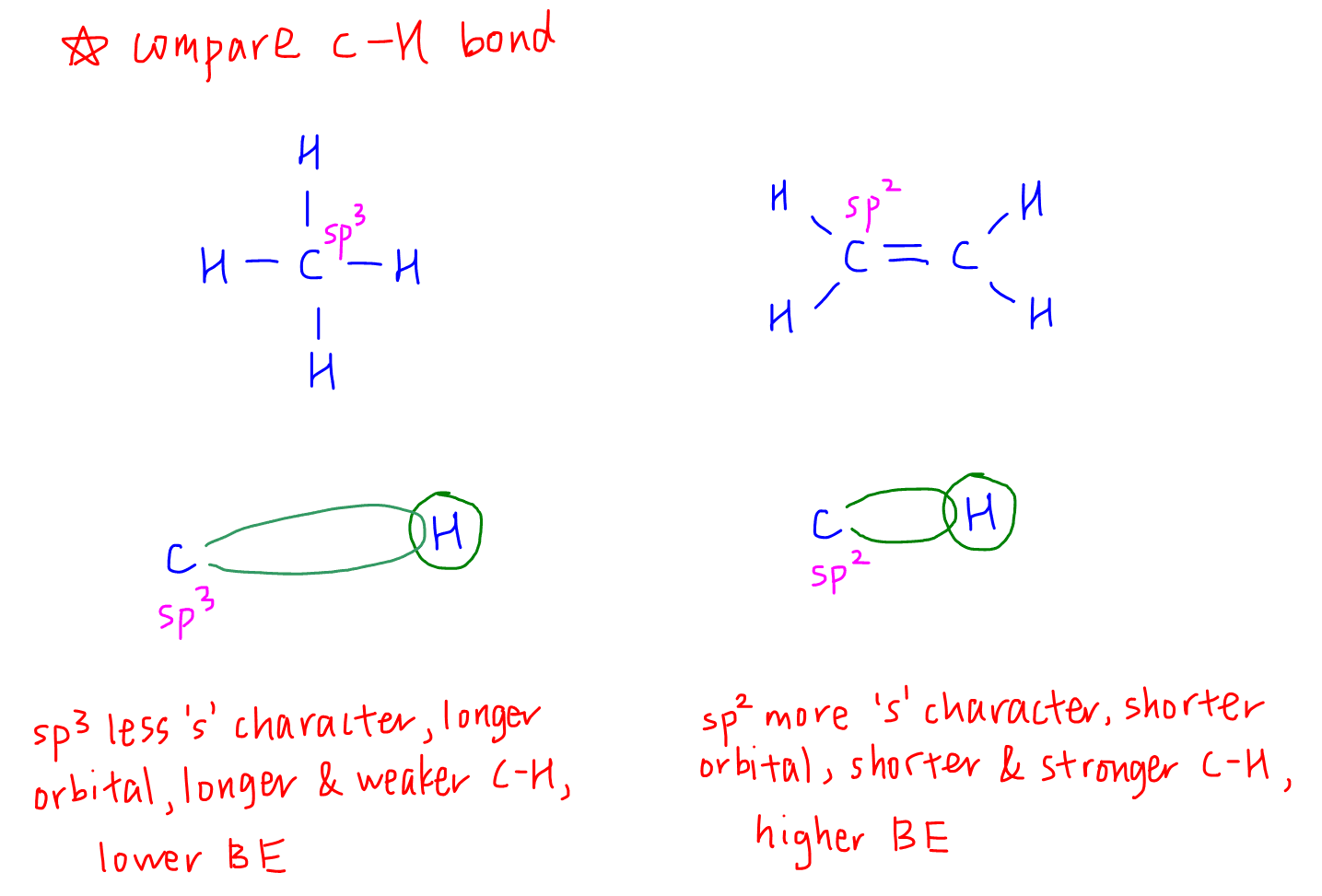
Carbon in methane is sp3 hybridised, so its orbitals are longer. The C-H bond in methane will be weaker and has lower bond energy.
Carbon in ethene is sp2 hybridised, so its orbitals are shorter. The C-H bond in ethene will be stronger and has higher bond energy.
2. Compare C-C bond
Let's have another example to compare C-C bonds.
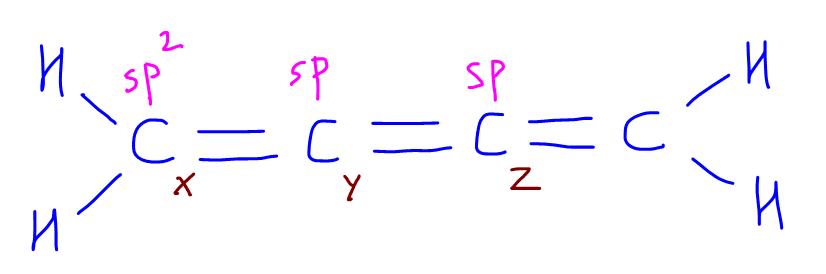
If we want to compare C-C double bond between Cx-Cy and Cy-Cz, we would again need to consider their state of hybridisation.
Cx is sp2 hybridised and the sp2 orbital is slightly longer while Cy and Cz are sp hybridised and the sp orbitals are slightly shorter.
Therefore Cx-Cy will have a longer and weaker bond with lower bond energy, and Cy-Cz will have a shorter and stronger bond with higher bond energy.
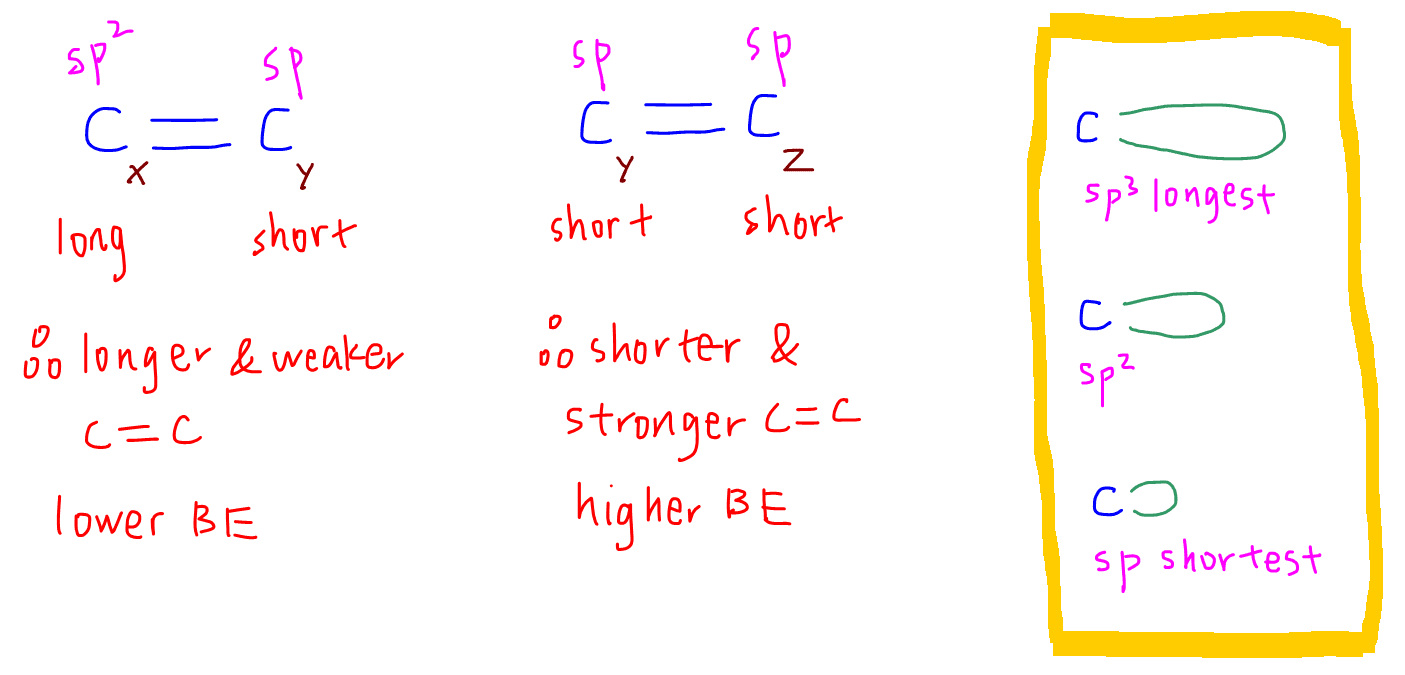
Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
