Condensation Polymerisation of Polyesters
Polyesters are a type of plastic made up of simpler units or monomers held together via ester bonds.
Let us first consider the condensation reaction of carboxylic acid and alcohol to form ester.
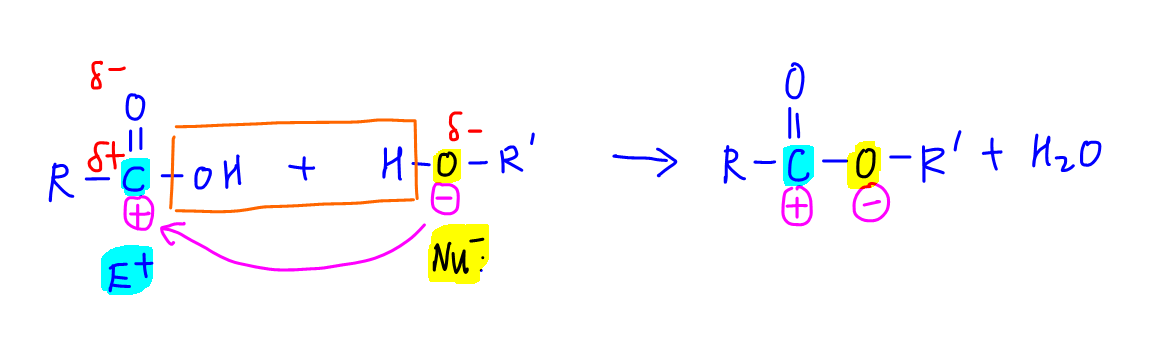
During esterification, the oxygen in alcohol acts as a nucleophile and attacks the acid carbon that acts as an electrophile.
The products formed are ester and water.
For one acid and one alcohol, we can only get one ester and one water.
If we have a di-acid and di-ol, something interesting can happen.
The di-acid can form 2 ester bonds with 2 different di-ols on either side.
Similarly the di-ol can also form 2 ester bonds with 2 different di-acids on either side.
This will form a continuous chain of alternating di-acid and di-ol, linked together via ester bonds.
Hence this polymer is called a polyester, made up of monomers di-acid and di-ol.
The balanced equation for this polymerisation is as shown:
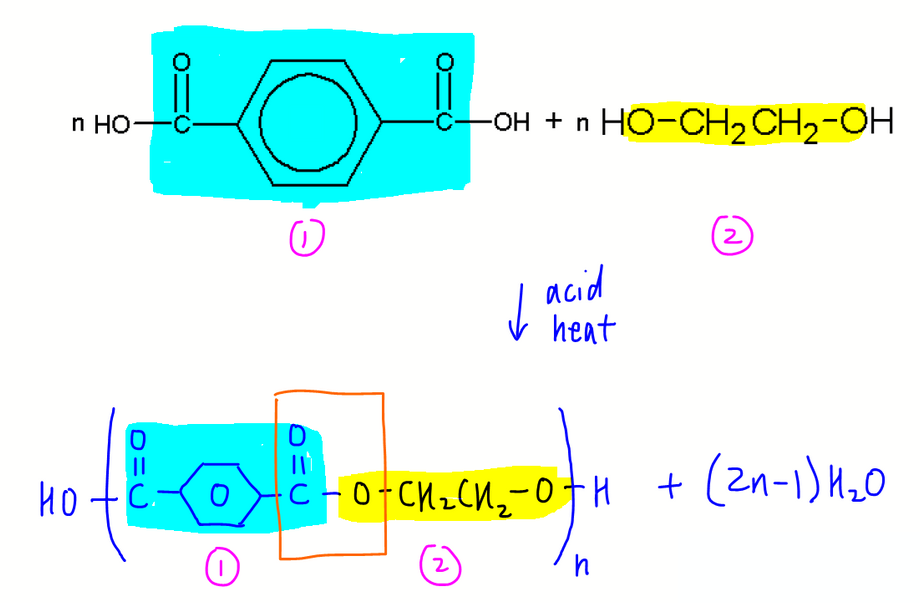
Notice n moles of di-acid and n moles of di-ol will give 2n-1 moles of water instead of 2n moles.
This difference in 1 water is due to an -OH group at the beginning and a -H group at the end of the polyester chain.
Topic: Carboxylic Acid and Derivatives, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
