Purification of Copper via Electrolysis
Electrolysis can be used to purify impure copper that contains other metals such as silver, gold, iron and zinc.
The purity of copper can be increased from 99% in impure copper to almost 99.99% in pure copper.
This purification process via electrolysis is important as slight amounts of impurity in copper will reduce its electrical conductivity.
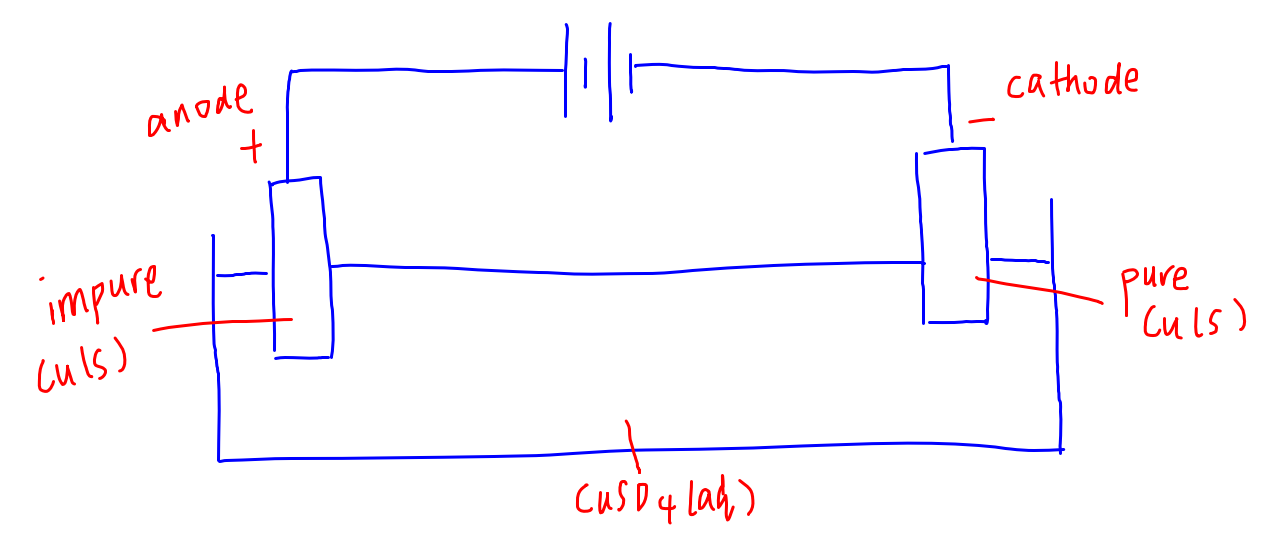
Electrolyte: copper (II) sulfate solution
Anode: impure copper
Cathode: pure copper
1. Oxidation at Anode (impure Copper)
At the anode we have to consider the oxidation of sulfate, water and all the metals in impure copper.
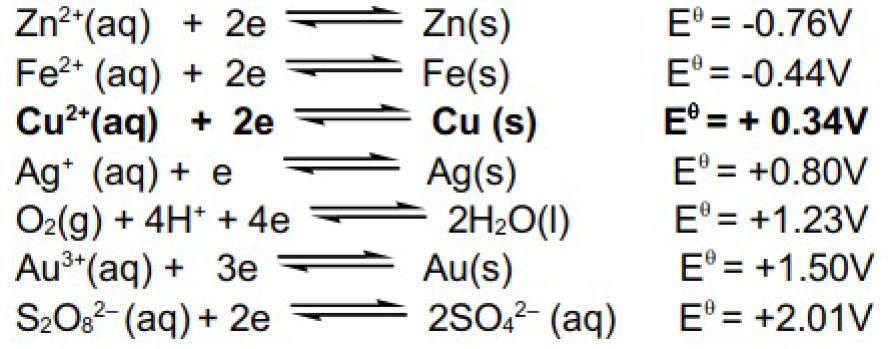
In this example zinc has the most negative E value hence will be oxidised first.
Zn(s) → Zn2+(aq) + 2e
Once zinc is depleted, the next metal to be oxidised will be iron with the next most negative E value.
Fe(s) → Fe2+(aq) + 2e
Once iron is depleted copper will be oxidised.
Cu(s) → Cu2+(aq) + 2e
Since the percentage of copper in the anode is already very high, oxidation of copper will take place for most of the electrolysis process.
Less reactive metals such as silver and gold will not be oxidised and they will be removed as sludge below the anode.
2. Reduction at Cathode (pure Copper)
At the cathode we have to consider the reduction of Cu2+, Zn2+, Fe2+ and water.
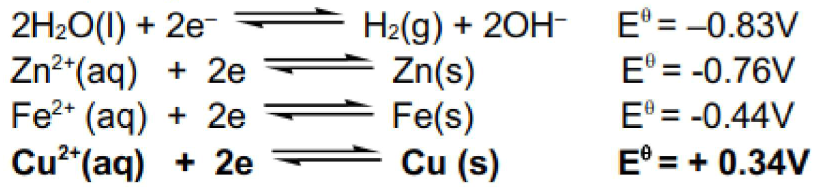
Cu2+ has the most positive E value hence will be reduced and deposited at the pure copper cathode.
Cu2+(aq) + 2e → Cu(s)
Cu2+ concentration will remain relatively high and constant throughout the electrolysis since Cu2+ is formed at the anode.
Zn2+ and Fe2+ will not be reduced and remain as ions in the solution.
Topic: Electrochemistry, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
