2019 A Level H2 Chemistry Paper 1 Question 8 - Deduce Germanium Properties from Period 3 Elements
This is a pretty straightforward question - 2019 A Level H2 Chemistry Paper 1 Question 8.
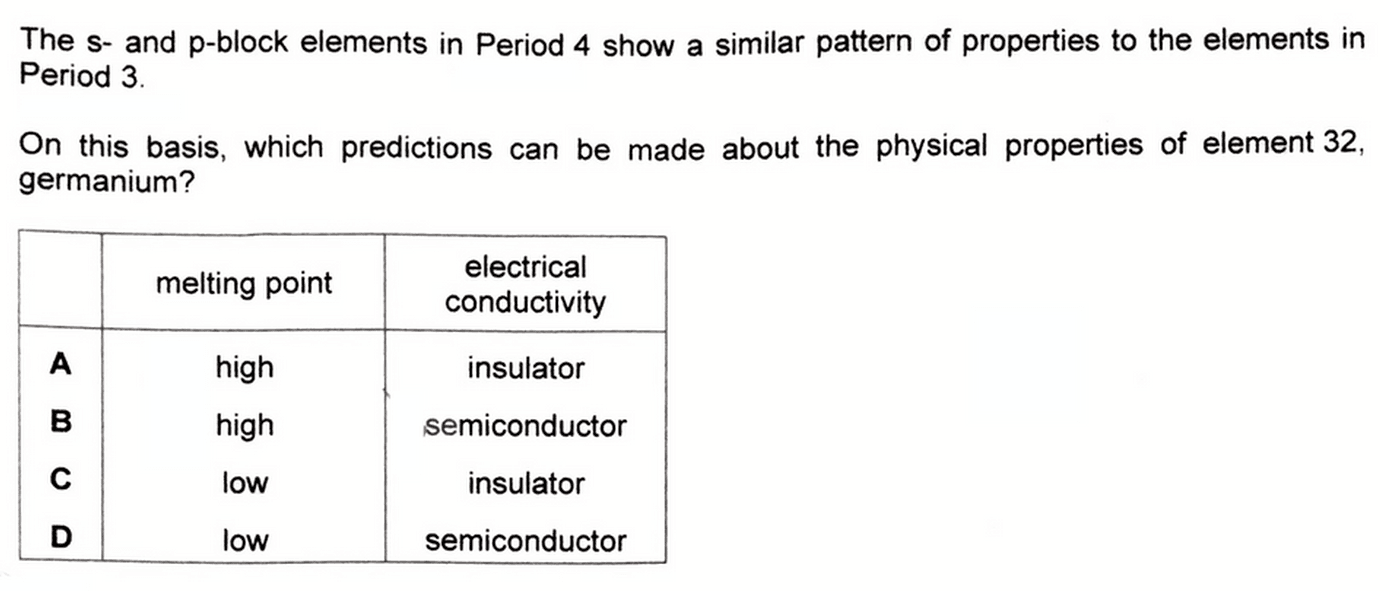
Given the properties for elements in Period 4 and Period 3 are similar, we are required to deduce the physical properties of germanium.
First we will have to determine which Group germanium is in.
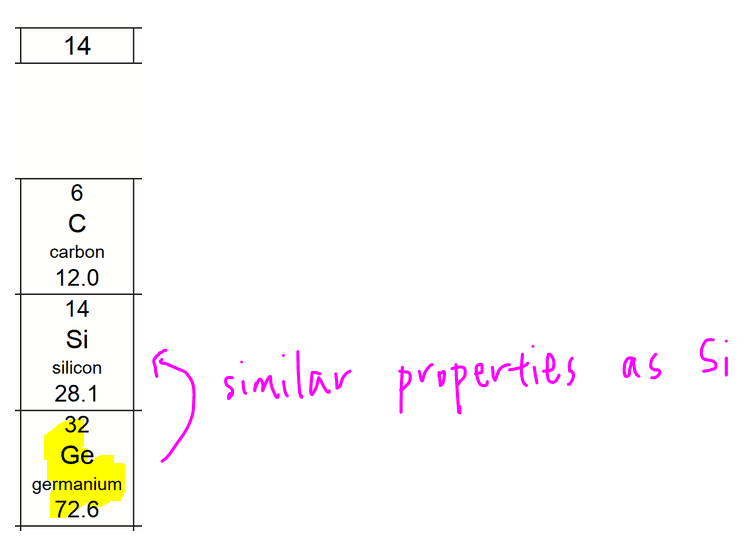
From the Periodic Table we can find germanium under Group 14, which is in the same Group as carbon and silicon.
So we will expect germanium (Period 4) and silicon (Period 3) to have similar properties.

Looking at the options we will expect both germanium and silicon to have high melting points and act as semiconductors.
Therefore the answer to this question will be option B.
Topic: Periodicity, Inorganic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
