How to Deduce Intermolecular Force for Simple Molecule
Here is the recommended sequence to deduce the intermolecular force (IMF) of a simple molecule:
1. Is H-F, H-O or H-N bond present?
If yes, its IMF will be hydrogen bonds. If not, consider the next point.
2. Are there any polar bonds?
A bond is polar when an electronegative element (F, O, N, Halogens, S) is bonded to a non-electronegative element.

If no, its IMF will be instantaneous dipole - induced dipole (id-id) attractions.
Examples of non-polar molecules with non-polar bonds are elements and hydrocarbons.

If yes, consider the next point.
3. Consider the shape of molecule. Do the dipole moments cancel?
If the dipole moments do not cancel out exactly, the molecule is polar and IMF will be permanent dipole - permanent dipole (pd-pd) attraction.
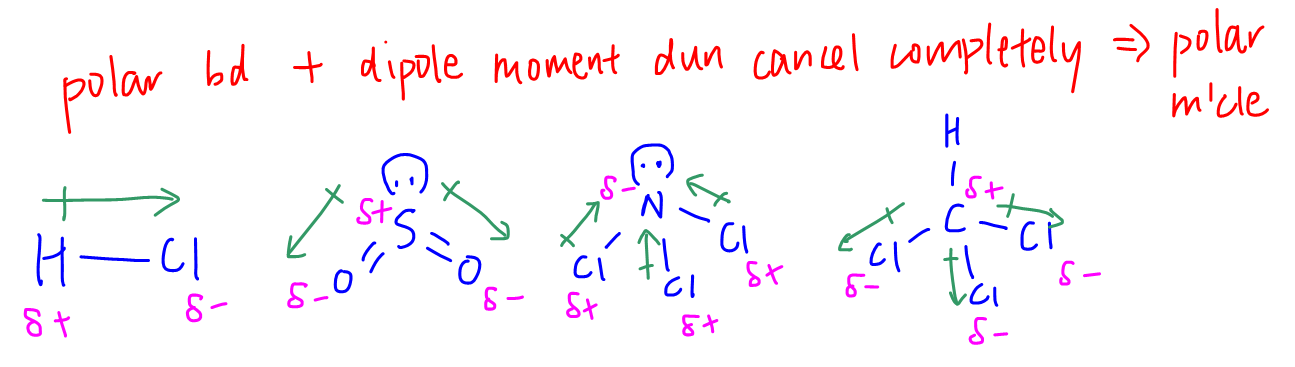
If the dipole moments cancel out exactly, the molecule is non-polar hence IMF will be id-id attraction.
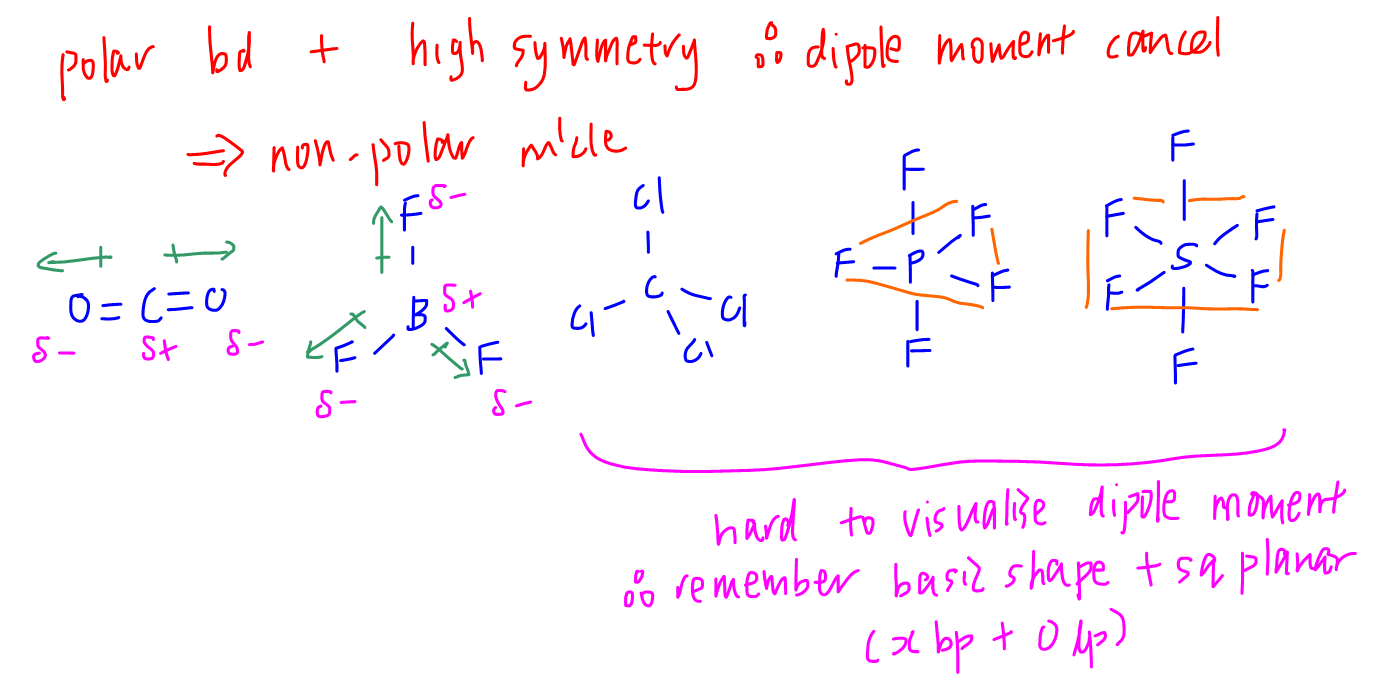
Sometimes it can be difficult to visualise the dipole moments and deduce if they cancel out or not.
Another way is to memorise the shapes that have high symmetry, the dipole moments will cancel out exactly and therefore the molecule is non-polar.
The shapes with high symmetry are basic shapes (linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral) and square planar.
Topic: Intermolecular Forces, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
