How to Deduce Major Product when Markovnikov Rule Fails
In this video lesson created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to determine the major product formed when bromine water is added to the following compound.

There are 2 reactions to consider.
1. Electrophilic substitution of phenol
Since -OH group is highly activating, benzene will be very reactive and can undergo trisubstitution of bromine at positions 2, 4 and 6 with respect to -OH group.
Check out this video lesson to learn how to compare electrophilic substitution of benzene with -OH group and other substituents.
2. Electrophilic addition of alkene
With aqueous bromine we are adding Br and OH groups to alkene.
Since the alkene is asymmetrical we will have to determine the major product.
Usually we will be using Markovnikov Rule to deduce major product when hydrogen is added eg HCl, HBr, H2O.
Markovnikov Rule states that hydrogen will be added to the alkene carbon with more hydrogen to form the major product.
Learn all about Markovnikov Rule here.
Since we are not adding hydrogen then we can consider modified Markovnikov Rule instead, where the electrophile is added to the alkene carbon with more hydrogen to form major product.
Check out what is modified Markovnikov Rule.
However in our compound, both alkene carbons have the same number of hydrogen, hence Markovnikov Rule is not useful to predict major product.
Let's consider the electrophilic addition mechanism and compare the stability of carbocations formed.
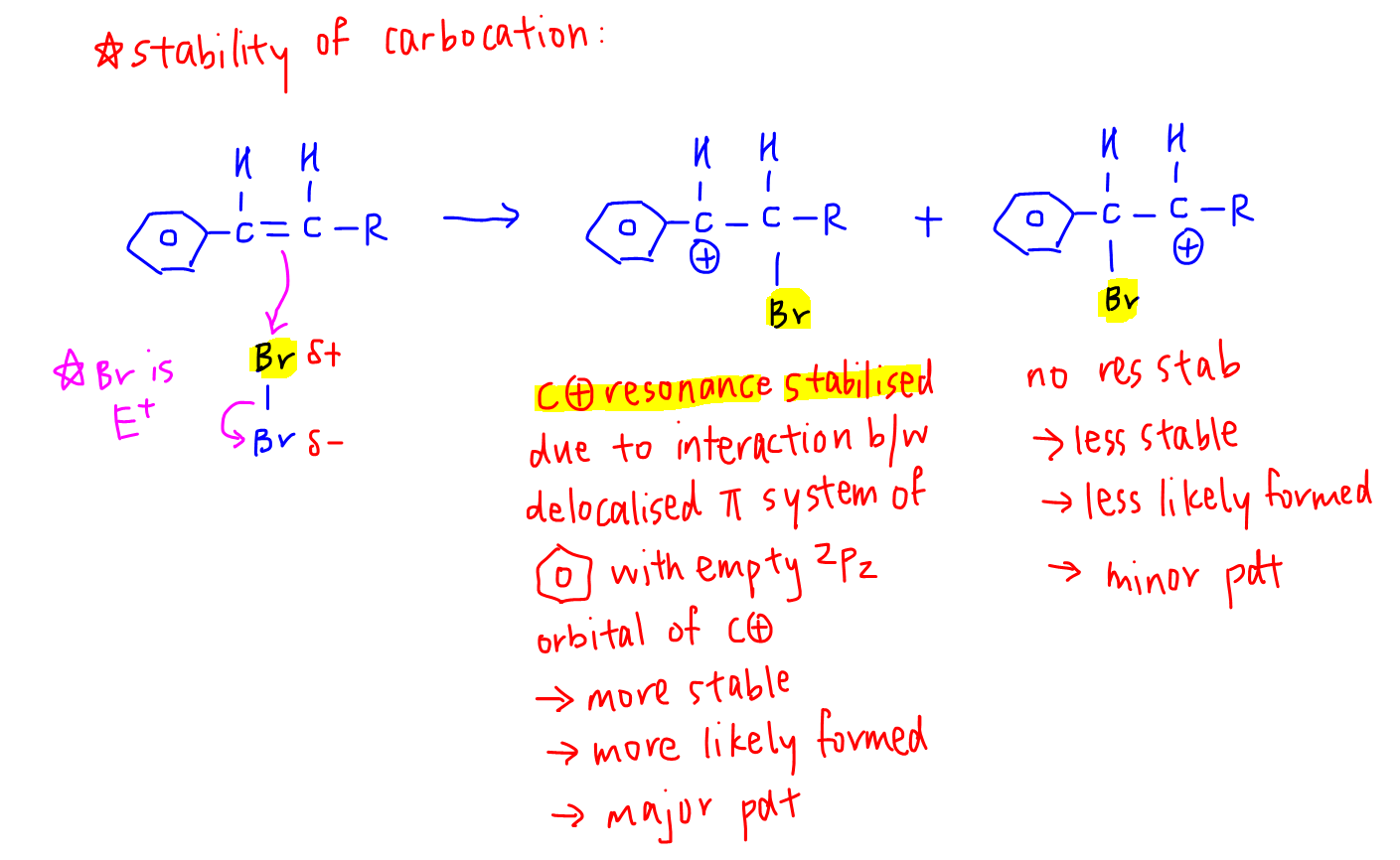
Notice when the carbocation is next to benzene, the delocalised pi system of benzene can interact with the empty p orbital of carbocation.

The carbocation is resonance stabilised and significantly more stable than the other carbocation.
Hence it will be more likely formed and give us the major product.

Remember when both alkene carbons have the same number of hydrogen, Markovnikov Rule fails.
In this case one of the alkene carbon will most likely be bonded to benzene.
To form the resonance stabilised carbocation, we will place the C+ next to benzene and bromine to the other carbon.
Finally OH- will join to the carbocation to form our major product.
Topic: Alkenes, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
