Deduce Species with Paramagnetism

Paramagnetic species contain unpaired electrons.
Hence we will need to write out the electronic configuration of these 4 species to determine which has unpaired electrons.
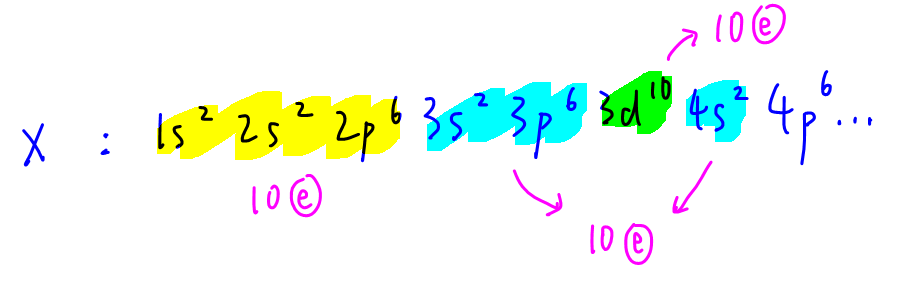
The electronic configuration for the first 30+ electrons are as shown:
1. O
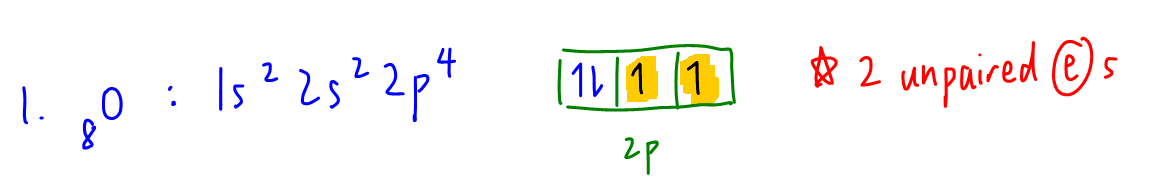
Notice oxygen has 4 electrons in 2p subshell but when we write out the electron in box diagram there are 2 unpaired electrons.
Hence O will be paramagnetic.
2. Al+
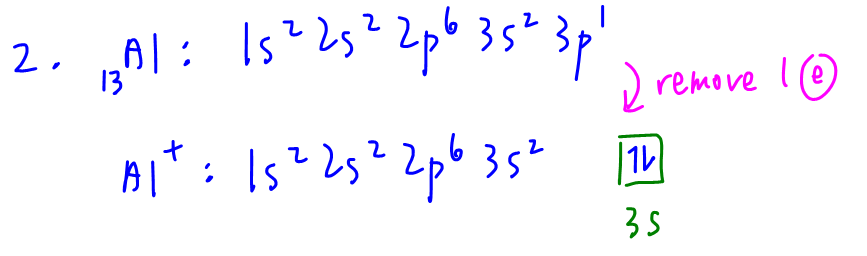
The correct way to determine electronic configuration of cation is to write out electronic configuration of neutral atom, then remove electrons from valence subshells to form the required cation.
Valence 3s subshell has 2 electrons that are paired, hence Al+ is not paramagnetic.
3. Ti2+
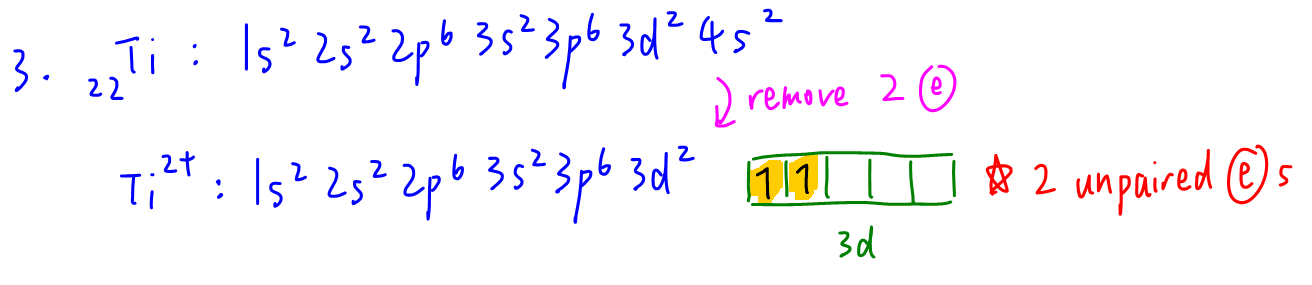
There are 2 unpaired electrons in 3d subshell hence Ti2+ is paramagnetic.
4. Cu+
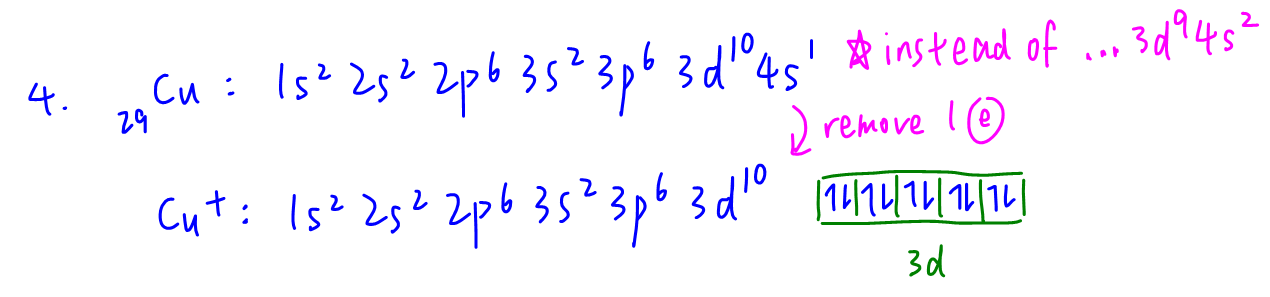
Notice there is an anomaly in writing electronic configuration of copper.
Copper prefers ...3d10 4s1 instead of ...3d9 4s2 configuration due to additional stability from fully filled 3d subshell.
The 3d subshell in Cu+ is fully filled with no unpaired electrons hence Cu+ is not paramagnetic.
Since only species 1 and 3 exhibit paramagnetism, answer to this question will be option A.

Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
