Deduce Type of Organic Chemistry Reaction
Here's the question for this week:
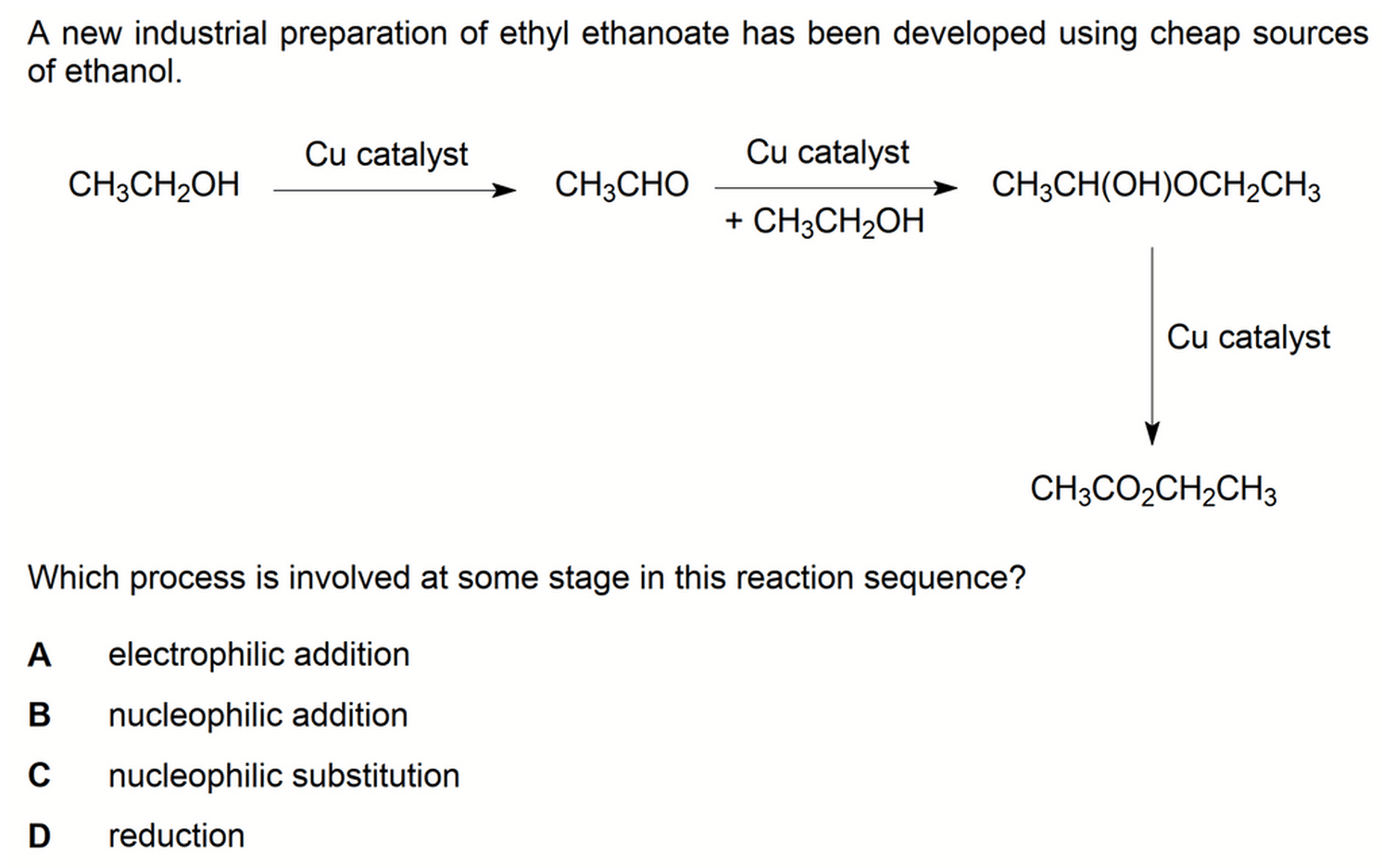
We have to deduce the type of reaction for each stage since all these reactions are not in syllabus.
This can be done by comparing molecular/structural formula of the reactants and products in detail.
Let's consider this step by step.
Step 1 - Oxidation
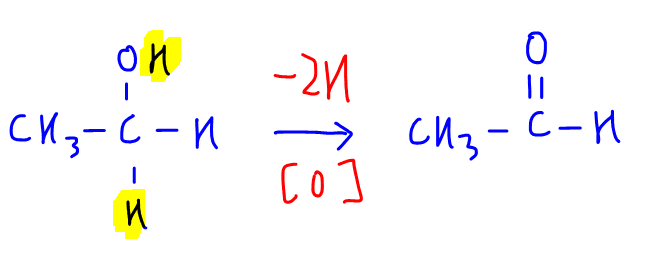
Notice this step involves losing 2 hydrogen.
Hence this step is oxidation, which is the loss of hydrogen, gain of oxygen or increase in oxidation state.
Check out how to calculate oxidation state of carbon in organic compounds.
Step 2 - Nucleophilic Addition
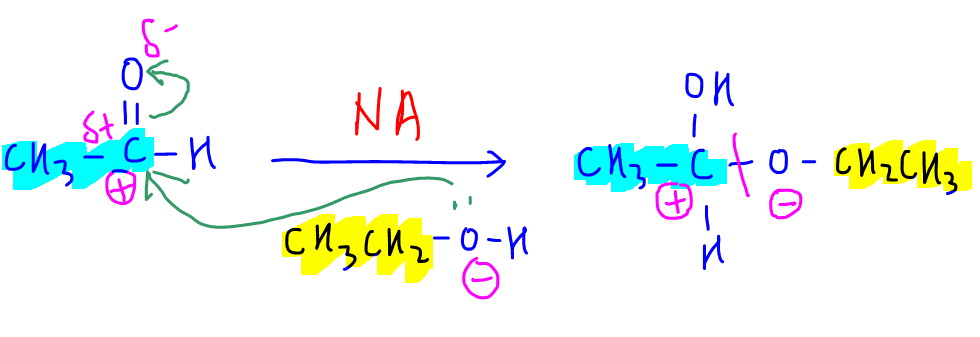
The reactants are ethanal (2 carbon) and ethanol (2 carbon) to form product with 4 carbons and 2 oxygen.
Therefore we can deduce oxygen in ethanol acts as a nucleophile and attacks carbonyl carbon in ethanal.
This results in an addition product, hence the type of reaction is nucleophilic addition.
Learn how to draw the mechanism of nucleophilic addition of carbonyl compounds.
Step 3 - Oxidation
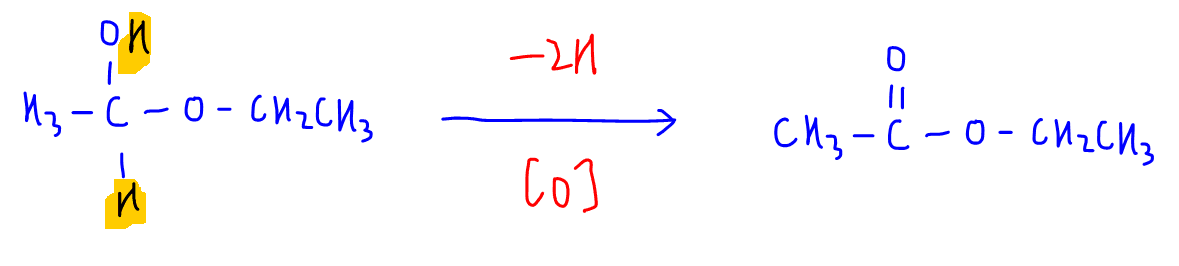
This step is very similar to the first step where the reactant loses 2 hydrogen to form ester product.
Hence this step is oxidation.
Finally we can compare the options and determine the answer to this question is option B (nucleophilic addition).

Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
