Deduce Zwitterion and Isoelectric Point of Amino Acids
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, we want to learn how to draw the zwitterion and calculate the isoelectric point of an amino acid.
Let's have these few amino acids as examples: alanine, cysteine and lysine.

1. Alanine
A zwitterion is charged but overall neutral.
We can deduce this by considering the acid-base reaction between the alpha acid and alpha amino group.
Alpha acid will donate proton to form conjugate base -COO-
Alpha amine will accept proton to form conjugate acid -NH3+

Next we have to deduce the pH range where the zwitterion is formed.
We have to look at each functional group and deduce the pH range for that functional group.
Alpha acid has reacted to form -COO-, hence solution is alkaline with respect to alpha acid, pH greater than pKa1 (2.35).
Alpha amine has reacted to form -NH3+, hence solution is acidic with respect to alpha amine, pH less than pKa2 (9.87).
We can use a number line to help visualise the overall pH range.

Hence the pH range where alanine exists predominantly as zwitterion is between pH 2.35 to 9.87.
Finally we can calculate the isoelectric point where the amino acid exists 100% as zwitterion.
Isoelectric point is the midpoint of the pH range that we have deduced previously.

Therefore isoelectric point of alanine is 6.11.
2. Cysteine
For cysteine we have a thiol (-SH) functional group in the R group.
Take note even though the pKa of thiol is 8.00, it is acidic in nature.
Let us consider the acid-base reaction to form zwitterion.
Alpha amine is the only base present hence will accept proton to form conjugate acid -NH3+
Both alpha acid and thiol groups are acidic, but alpha acid is stronger so it will be the proton donor to form -COO-

Therefore an easy way to deduce the zwitterion is to consider the acid-base reaction between the strongest acid and strongest base in the amino acid.
Next we have to deduce the pH range where the zwitterion is formed.
Alpha acid has reacted to form -COO-, hence solution is alkaline with respect to alpha acid, pH greater than pKa1 (2.05).
Thiol remains unchanged, hence solution is acidic with respect to thiol, pH less than pKa2 (8.00).
Alpha amine is reacted to form -NH3+, hence solution is acidic with respect to alpha amine, pH less than pKa3 (10.25).

Notice the number line is very useful here to determine the pH range as there are 3 functional groups to consider.
Hence the pH range where cysteine exists predominantly as zwitterion is between pH 2.05 to 8.00.
Finally we can calculate the isoelectric point for cysteine.

Therefore isoelectric point of cysteine is 5.02.
3. Lysine
For lysine we have an amine functional group in the R group.
Let us consider the acid-base reaction between the strongest acid and strongest base to form zwitterion.
Alpha acid is the only acid hence will react to form -COO-
R group amine, with a larger pKa value, is the stronger base, hence will accept proton to form conjugate acid -NH3+
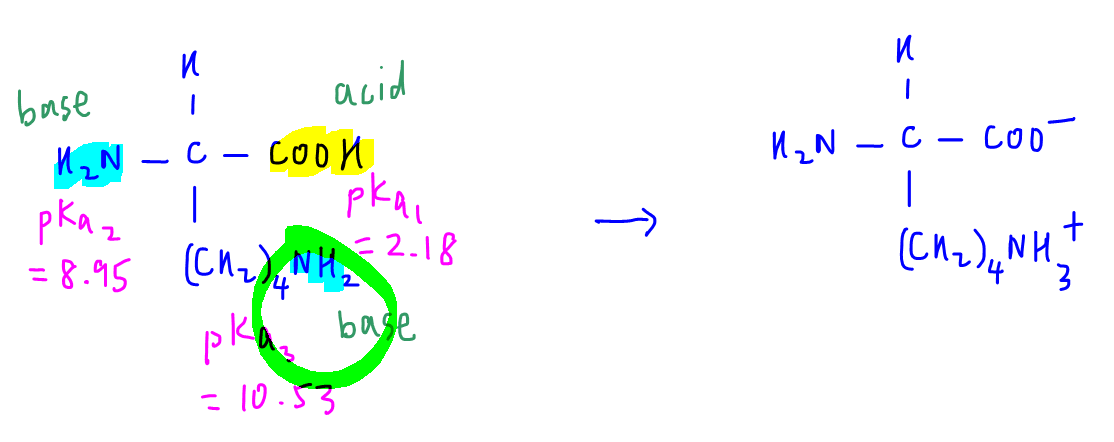
Next we have to deduce the pH range where the zwitterion is formed.
Alpha acid has reacted to form -COO-, hence solution is alkaline with respect to alpha acid, pH greater than pKa1 (2.18).
Alpha amine is unreacted, hence solution is alkaline with respect to alpha amine, pH greater than pKa2 (8.95).
R group amine has reacted to form -NH3+, hence solution is acidic with respect to R group amine, pH less than pKa3 (10.53).

Using the number line, we can easily determine the pH range where lysine exists predominantly as zwitterion to be between pH 8.95 to 10.53.
Finally we can calculate the isoelectric point for lysine.

Therefore isoelectric point of lysine is 9.74.
Topic: Nitrogen Compounds and Proteins, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
