Determine Enthalpy Change of Reaction between Sodium and Water

To make it easier to deduce which reactions are needed, we can write down the equations that correspond to each enthalpy change first.
1. Combustion of H2(g)
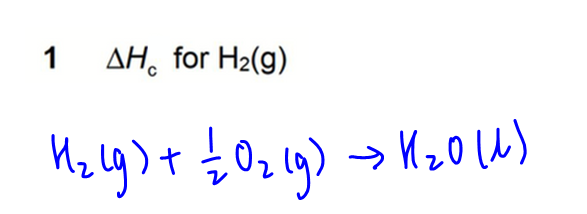
2. Combustion of H2O(l)
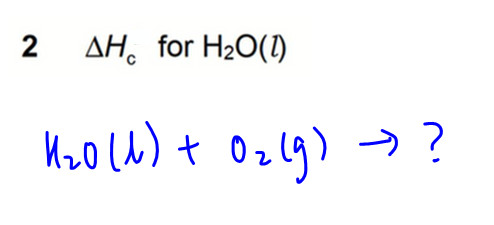
Notice water cannot be further oxidised since hydrogen has highest oxidation state of +1.
3. Formation of NaOH(aq)
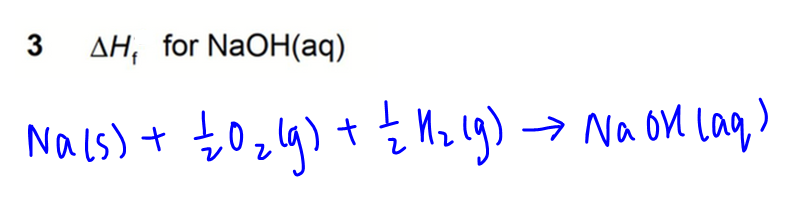
4. Combution of Na(s)
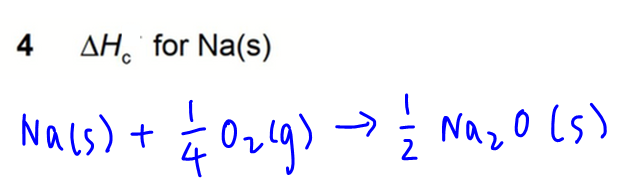
Next is to draw the energy cycle linking reaction 1 and some of the above-mentioned processes.
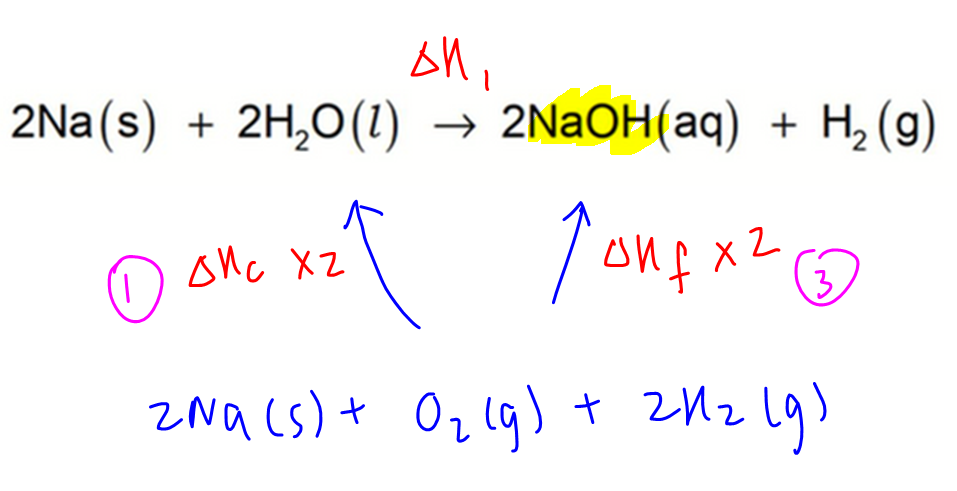
Therefore answer to this question is option B.
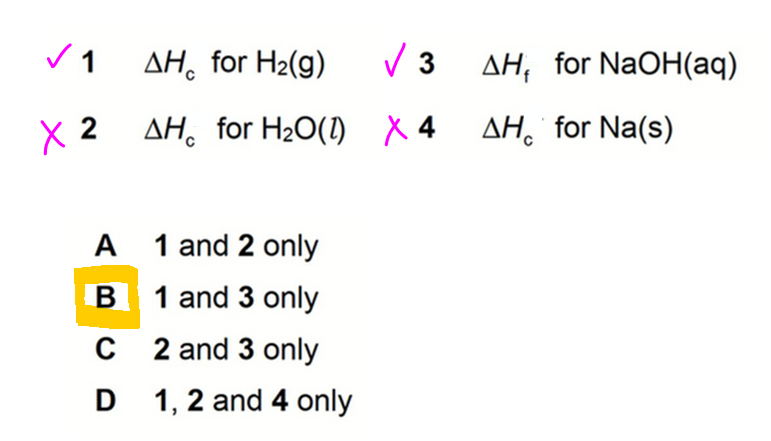
Topic: Energetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
