Determine Moles of Electrons Gained per Mole of Oxidising Agent

Let's put down all the information we have from the question so far.
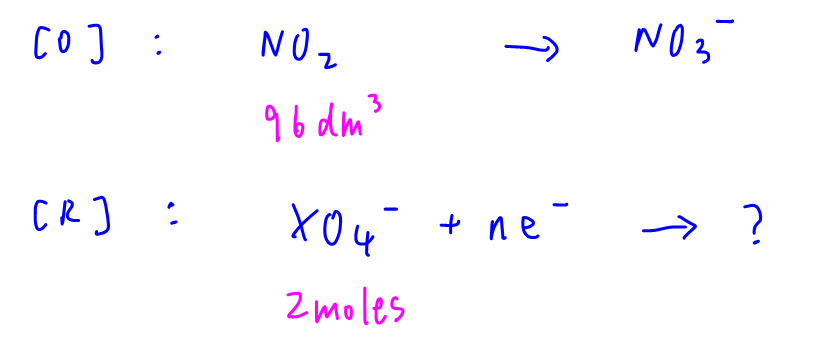
96 dm3 of NO2 is oxidised to NO3- while 2 moles of XO4- is reduced.
These are the recommended 4 steps to determine oxidation state of unknown species from experimental data:
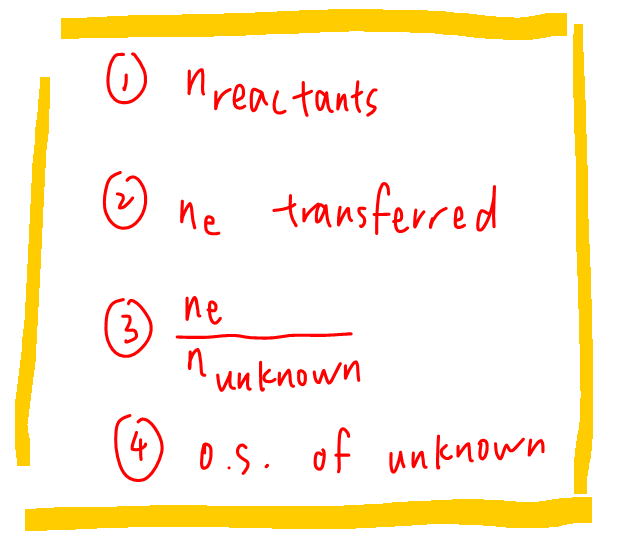
1. Determine moles of reactants
At room temperature and pressure, moles of NO2 = 96 / 24 = 4
Moles of XO4- = 2 (given)
2. Determine moles of electrons transferred in reaction
We need to balance the oxidation half equation of NO2 in acidic medium.

From half equation, mole ratio of electron lost to NO2 is 1 to 1.
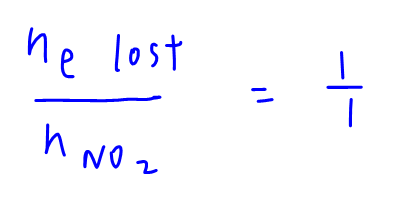
Hence mol of electron lost by NO2 = mol of NO2 = 4 = mol of electron gained by XO4-
This step is fundamental since we know redox reaction is a total transfer of electrons from reducing agent to oxidising agent, so moles of electrons involved in oxidation of NO2 and reduction of XO4- will be the same.
3. Determine mole ratio of electrons to XO4-
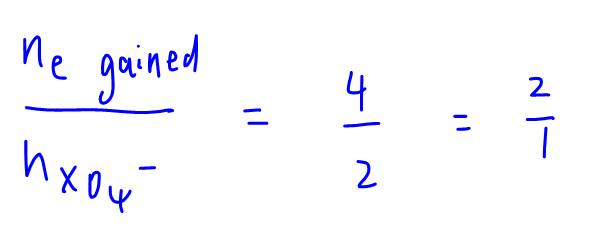
This basically says that each XO4- gains 2 electrons, so the answer to this question will be B.

The question didn't require us to find final oxidation state of X, but we can easily do that since we know the initial oxidation state of X and change in oxidation state.
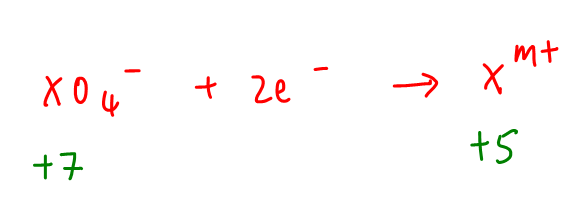
Initial OS of X in XO4- = +7
Change in OS = -2
Final OS of X in product = +7 - 2 = +5
Topic: Redox Titration, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
