Deduce Total Pressure on Mixing of Gases

When we have a few gases at different volumes and pressures, it is less straight forward to find the final pressure.
We can start with summing up the number of moles, since we know that the moles of gases in bulbs R and S will add up to the total moles of gas in the final mixture.
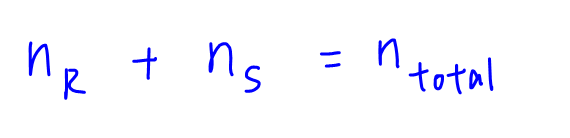
We can use the ideal gas equation and write the terms in terms of moles.

We can then substitute moles of R, S and total moles in terms of pressure, volume, gas constant R and temperature.
Since R and temperature are constant, these terms can cancel to give an expression in terms of pressure and volume.
We can then determine total pressure to be 125 kPa.
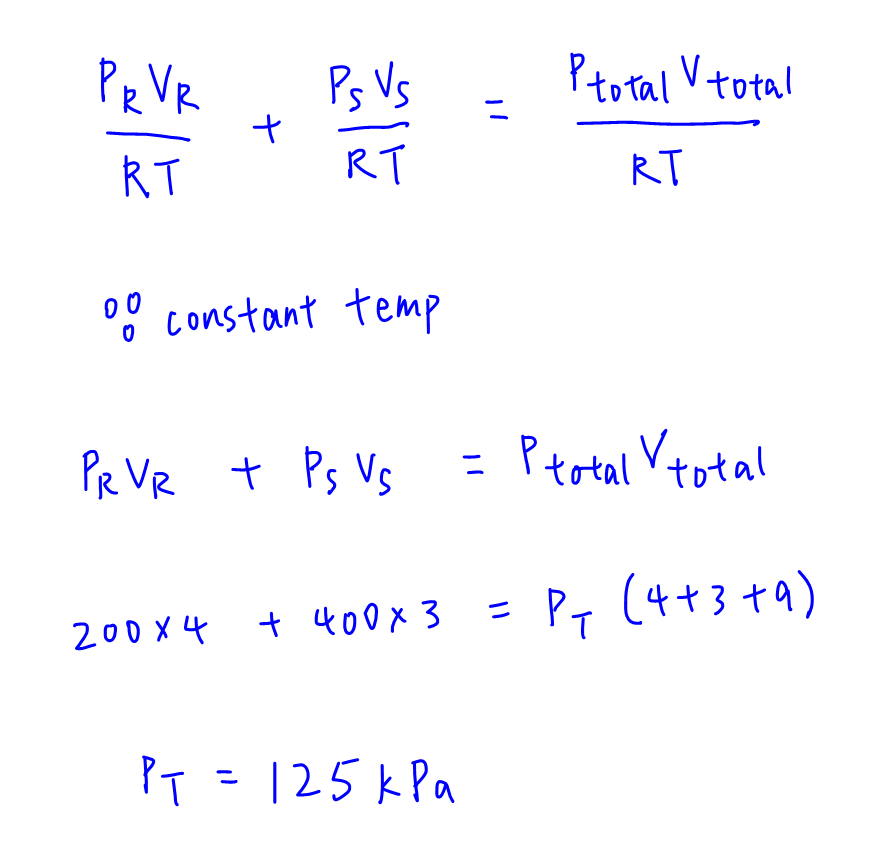
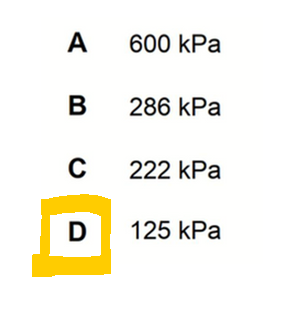
Hence answer to this question is option D.
We can memorise the following formula to determine final pressure when 2 gases with different pressure and volume are mixed together under constant temperature.

Topic: Gaseous State, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
