Determine Electrolysis Products via E Value
Let's predict the electrolysis products of dilute KOH using platinum electrodes.
The species to consider are:
Cathode: K+ and water
Anode: OH- and water

1. Reduction at Cathode
Since K+ and water can be reduced at the cathode, we have to use their electrode potentials or E value to compare their ease of reduction.
Species reduced are found on the left hand side of the half equations in the Data Booklet, so we focus on finding K+ and H2O on the left hand side of half equation.

Water has a more positive E value, more likely reduced hence will be reduced at the cathode.
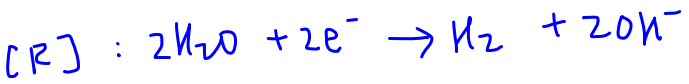
Product at cathode is H2 gas.
2. Oxidation at Anode
OH- and water can be oxidised at the anode and we can use E value to compare their ease of oxidation.
Species oxidised are on the right hand side of half equation, so we focus on finding OH- and H2O on the right hand side of half equation.

OH- has a more negative E value, more likely oxidised hence will be oxidised at the anode.

Product at anode is O2 gas.
Topic: Electrochemistry, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
