Determine Feasibility of Redox Reaction using Standard Electrode Potentials
In Electrochemistry we need to know how to use standard cell potential (Ecell) to predict thermodynamic feasibility of a redox reaction.
Let's use a mixture of acidified manganate MnO4- and hydrogen peroxide H2O2 as an example.
Since H2O2 can be both oxidised and reduced, we will determine what happens to MnO4- first.

Manganate is a strong oxidising agent and will be reduced.
Therefore we will expect to find MnO4- on the left hand side of the half equation in the data booklet, since all half equations are written in reduction form.
Since MnO4- is reduced, then hydrogen peroxide must be oxidised.
So we need to choose the half equation where H2O2 appears on the right hand side of the half equation.
1. Choosing the relevant half equations
For reduction of MnO4-, it has to be on the left hand side of the half equation.

In acidic medium, MnO4- will be reduced to Mn2+.
In neutral or alkaline medium, MnO4- will be reduced to MnO2.
So the half equation that we will use is the one where MnO4- is reduced to Mn2+ and E value is +1.52V.
For oxidation of H2O2, it has to be on the right hand side of the half equation.
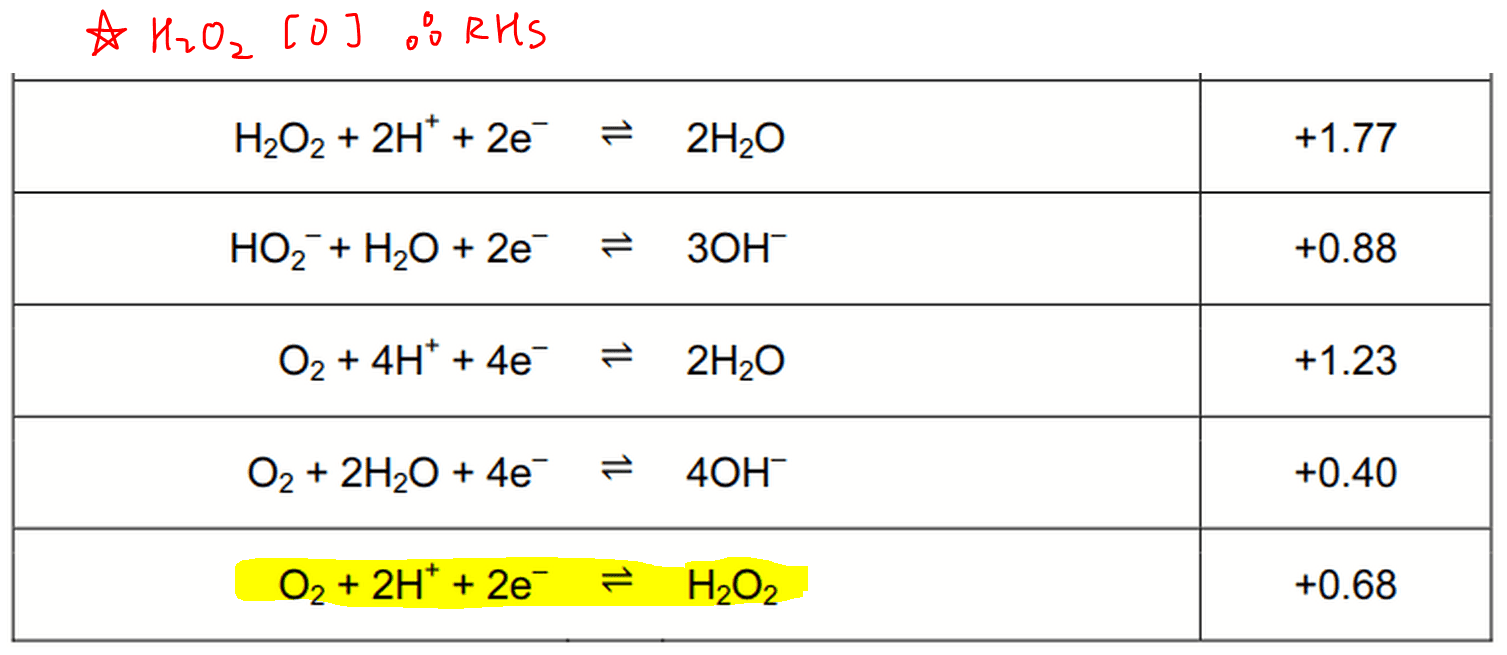
So the half equation that we will use is the one where H2O2 is on the right hand side, and O2 is on the left hand side and E value is +0.68V.
Notice there is another half equation where H2O2 is on the left hand side (E = +1.77V).
But we will reject that since H2O2 and MnO4- cannot be both reduced in the same reaction.
2. Determine Redox Reaction
With the 2 half equations chosen we now know that MnO4- is reduced while H2O2 is oxidised.

3. Calculate Feasibility
We can calculate standard cell potential or Ecell of this reaction using formula Ecell = ER - EO.

Since Ecell is a positive value, the reaction is thermodynamically feasible.
4. Balance Redox Reaction
If the half equations can be found in the data booklet, this is very simple as there is no need to use the half equation method to balance the redox equation.

Topic: Electrochemistry, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's renowned A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
