Determine Order of Reaction from Initial Rates
Comparing initial rates to determine the order of reaction is a very common question in Kinetics.
Another method is by plotting concentration of reactant or product against time graph and finding half life to determine order of reaction.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through an example:

The objective is to choose a pair of experiments for comparison where the concentration of a reactant changes and ideally the concentration of other reactants remain constant.
This means that any change in the initial rates of the experiments must be due to the change in the concentration of that reactant, and we can figure out the order from there.
1. Order of Reaction with respect to HCl
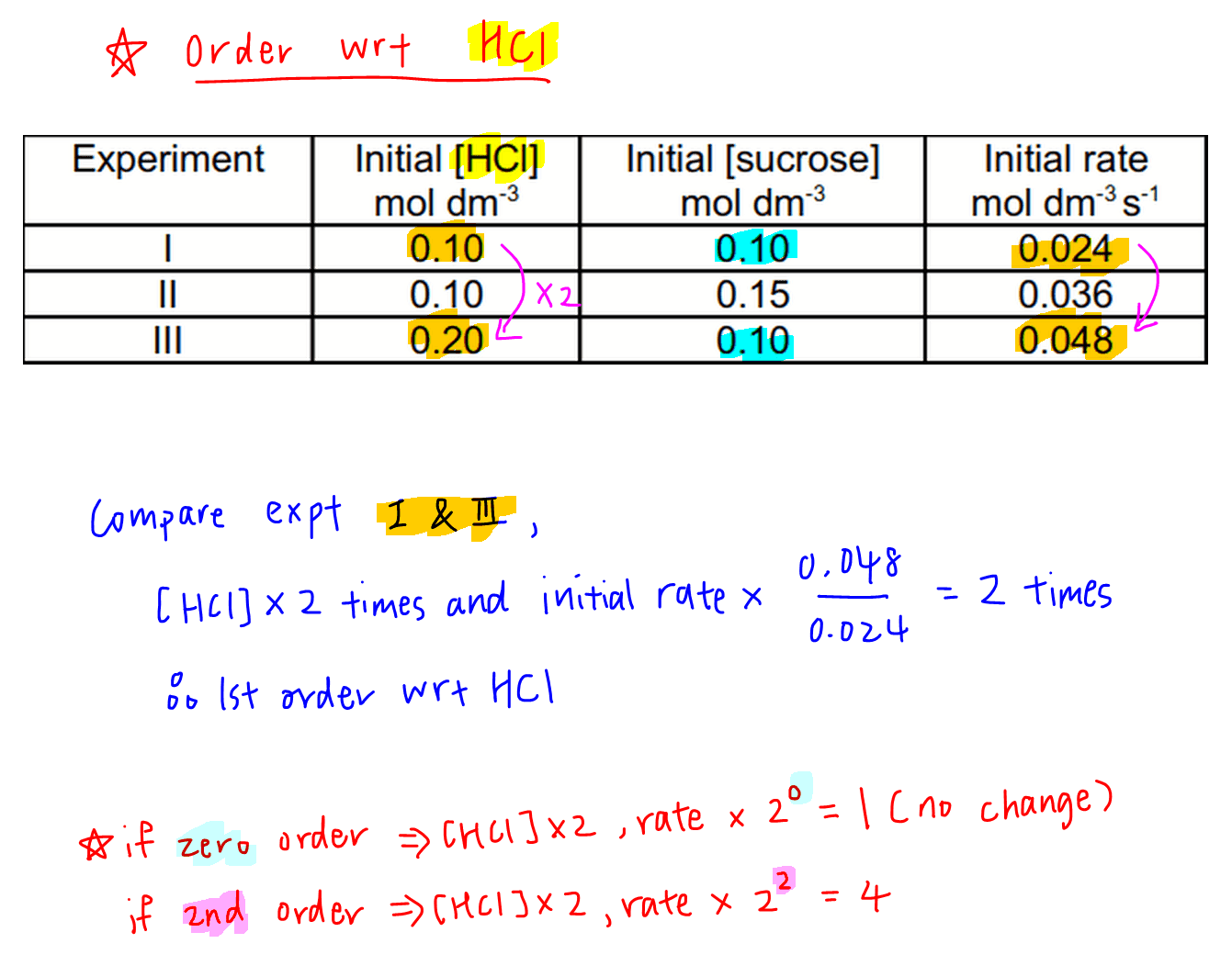
Comparing experiments 1 and 3, concentration of HCl doubles and there is no change in concentration of sucrose.
So the change in initial rates must be due to HCl only.
We can work out the change in initial rates to be 2 times.
This means when concentration of HCl doubles, initial rate doubles.
Therefore order of the reaction with respect to HCl will be order 1.
For comparison, if order of reaction is zero, initial rate will remain unchanged when concentration of HCl doubles.
If order of reaction is 2, initial rate will increase by 4 times (22 times) when concentration of HCl doubles.
Since we only have 3 possible orders to consider, figuring out the order of reaction is quite straightforward.
2. Order of Reaction with respect to sucrose
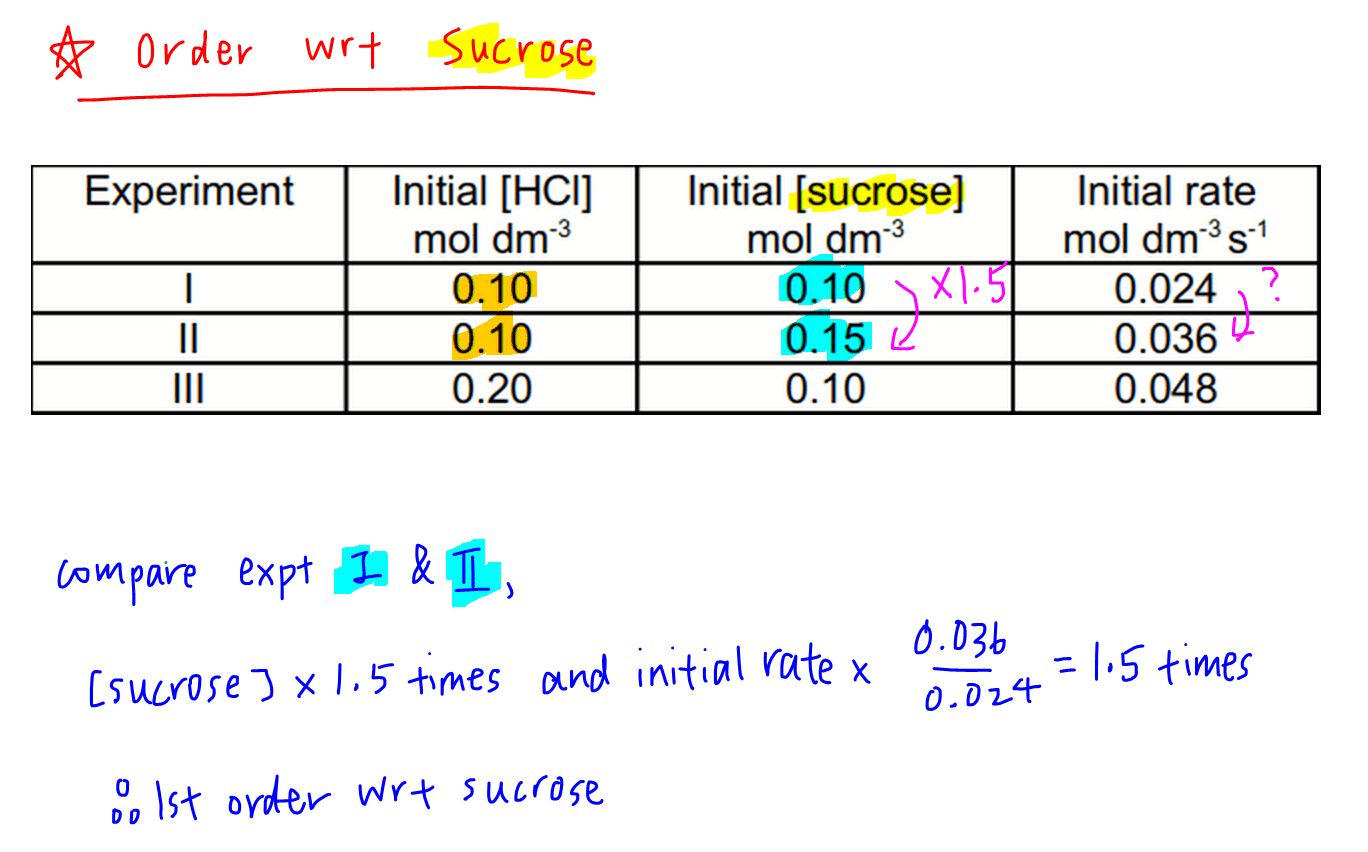
Comparing experiments 1 and 2, concentration of sucrose increase by 1.5 times and there is no change in concentration of HCl.
So the increase in initial rates by 1.5 times must be due to sucrose only.
Since this is a proportionate increase, order of reaction with respect to sucrose is also order 1.
Finally we can write out the rate equation for this reaction to be:
rate = k [HCl] [sucrose]
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's renowned A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
