Determine Oxidation State of Unknown Species from Experimental Data
Sometimes in redox questions we are required to determine the oxidation state of an unknown species.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through an example.

From the question we know that chlorine is reduced to chloride ions, and thiosulphate undergoes oxidation.
But the sulphur-containing product is not known, hence we need to determine the oxidation state of sulphur in the product, then decide which is the answer.
We can use the following 4 steps to determine oxidation state of an unknown species given experimental data.

Let's put these steps to work!
1. Determine number of moles of reactants
This is straightforward as the information will be available in the question for us to calculate amount of both reactants.

2. From known half equation, determine number of moles of electrons transferred
We can write out the 2 half equations first.

Notice the reduction half equation from Cl2 to Cl- can be balanced so the number of moles of electrons is known.
But for the oxidation half equation, since the product is unknown, we cannot balance this half equation and determine the moles of electrons.
We can use the known half equation to calculate the moles of electrons gained by Cl2 first.
Since electrons are totally transferred in a redox reaction, the moles of electrons lost by S2O32- will be equal to moles of electrons gained by Cl2.

3. Determine mole ratio of electron to unknown
We can now find mole ratio of electron lost to thiosulphate which will work out to be 8:1.

4. Determine oxidation state of unknown
Each S2O32- loses 8 electrons, and since there are 2 S in each thiosulphate ion, then each S will lose 4 electrons.
So the oxidation state of sulphur will increase by 4 units.
Finally we can determine oxidation state of S in S2O32- to be +2, and deduce that oxidation state of S in the product will be +6.
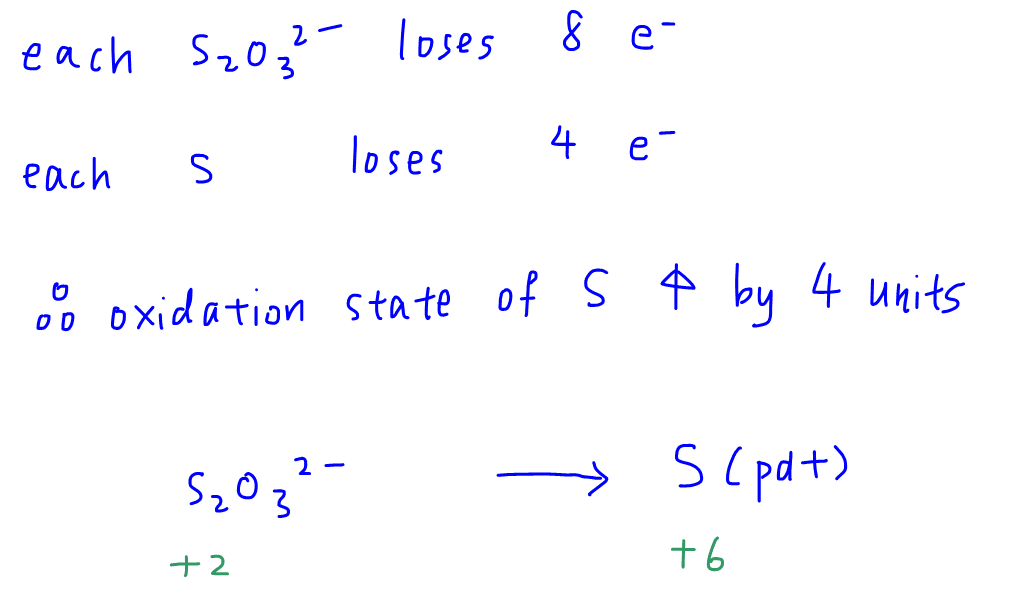
Comparing the options we can conclude the answer has to be option A where the oxidation state of sulphur in HSO4- is +6.

Topic: Redox Reactions, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's renowned A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
