2019 A Level H2 Chemistry Paper 1 Question 16 - Determine sp Hybridised Carbon in Organic Compound
Here's 2019 A Level H2 Chemistry Paper 1 Question 16.

We are required to determine which molecule contains 2 sp hybridised carbons.
First let's recap state of hybridisation of carbon.

sp3 hybridised carbon = 4 sigma bonds (4 single bonds)
sp2 hybridised carbon = 3 sigma bonds + 1 pi bond (2 single bonds + 1 double bond)
sp hybridised carbon = 2 sigma bonds + 2 pi bonds (either 2 double bonds or 1 single bond + 1 triple bond)
I have a previous video lesson on hybridisation of carbon in organic compounds so do check it out if you are interested.
So now we know how to deduce the state of hybridisation of carbon, let's run through each of the molecules.
A. Ethyne
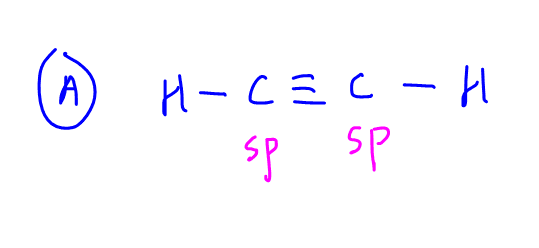
Both carbons are sp hybridised with 1 single bond + 1 triple bond for each carbon.
B. Ethene

Both carbons are sp2 hybridised with 2 single bonds + 1 double bond for each carbon.
C. 1,3-Butadiyne

All 4 carbons are sp hybridised.
D. 1,3-Butadiene

All 4 carbons are sp2 hybridised.
Since we want the molecule with 2 sp hybridised carbons, the answer to this question will be option A.
Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
