How to Sketch Titration Curve for Diprotic Acid
In this JC2 webinar we want to learn how to sketch titration curve for diprotic acid.
Let's consider the titration of ethanedioic acid (H2C2O4) with sodium hydroxide.
A diprotic acid will react with OH- in 2 stages:
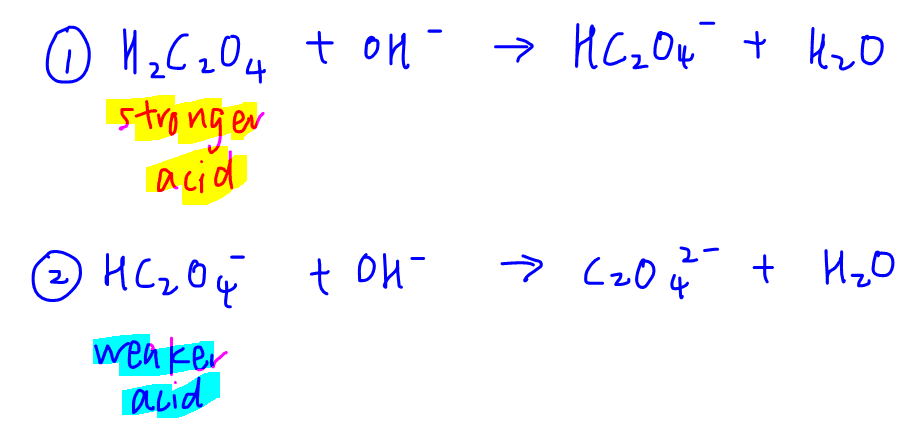
Each stage can be treated as a monoprotic acid-base reaction.
First acid H2C2O4 is stronger than second acid HC2O4-, hence reaction 1 will proceed first and go to completion before the second reaction will start.
At the end of each reaction, there will be an equivalence point.
Therefore we have 2 distinct reactions with 2 distinct equivalence points.
Here are the 3 points that we can use for plotting:
1. Initial pH
2. Maximum buffering capacity during reaction 1
3. Maximum buffering capacity during reaction 2
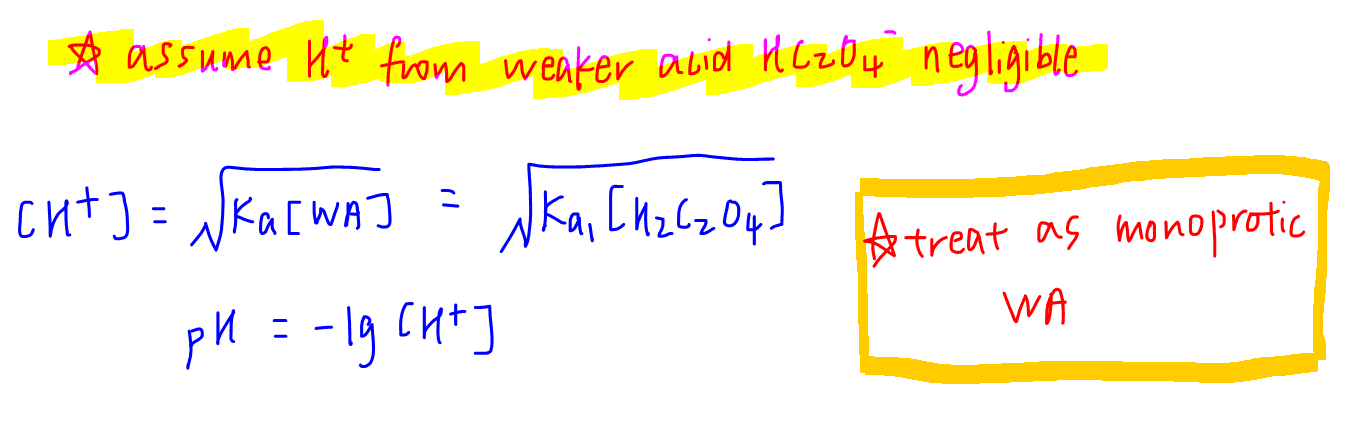
1. Initial pH
For a diprotic acid, we assume that the H+ only comes from the first dissociation of H2C2O4.
The second acid HC2O4- is weaker and will contribute an insignificant amount of H+.
So we can just treat a diprotic acid like a monoprotic acid, and focus on its first dissociation.
Hence the weak acid formula can be used to determine H+ concentration and pH.
2. Maximum buffering capacity during reaction 1
During reaction 1, H2C2O4 is in excess and a buffer is formed.
This buffering system is made up of weak acid H2C2O4 and conjugate base HC2O4-.
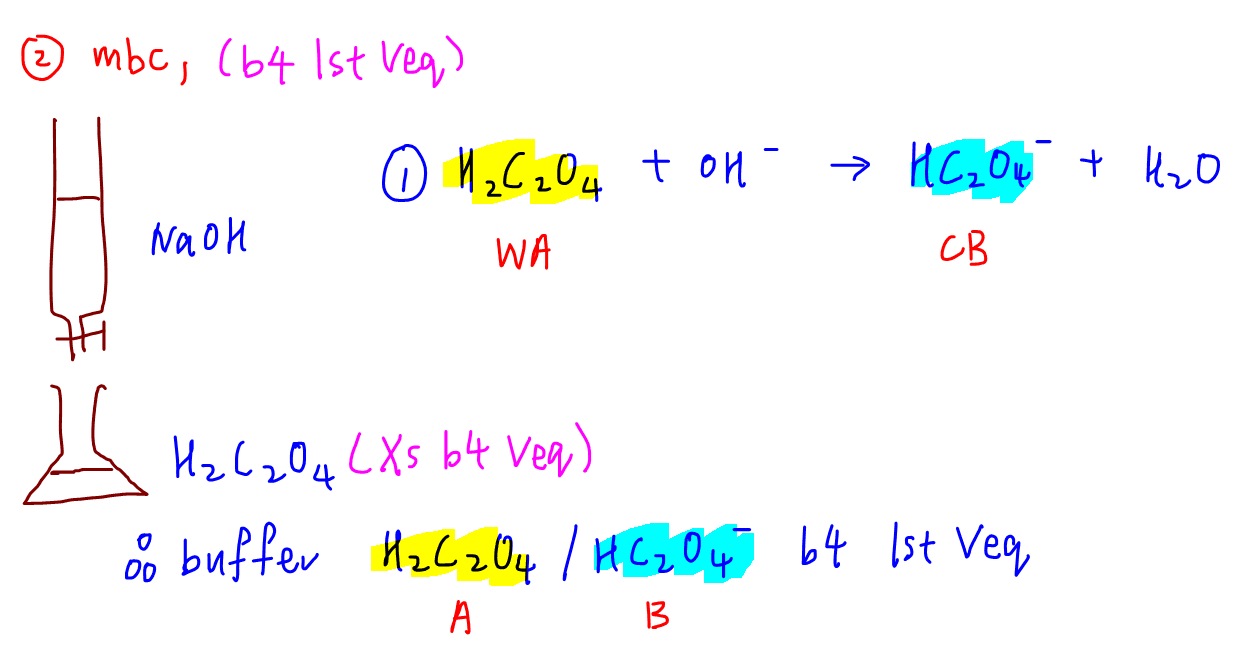
Hence we can determine its maximum buffering capacity for the first buffer region.

Mbc is very useful to plot on a titration curve since gradient at that point is zero.
Therefore the shape of the graph at and around mbc will be very well defined.
3. Maximum buffering capacity during reaction 2
Similarly, during reaction 2, HC2O4- is in excess and a buffer is formed.
This buffering system is made up of weak acid HC2O4- and conjugate base C2O42-.

Hence we can determine its maximum buffering capacity for the second buffer region.

Let's put everything together and plot our titration curve!
We can make use of the maximum buffering capacities to draw the buffer regions for both reactions.
The range of rapid pH changes at both equivalence points can then be added.

With these 3 points we can sketch a very decent looking titration curve for diprotic acids.
Topic: Buffer and Titration Curve, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's trusted JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
