2019 A Level H2 Chemistry Paper 1 Question 26 - Deduce Electronic Configuration of Mo(IV) Ion
Here's 2019 A Level H2 Chemistry Paper 1 Question 26 on transition elements.
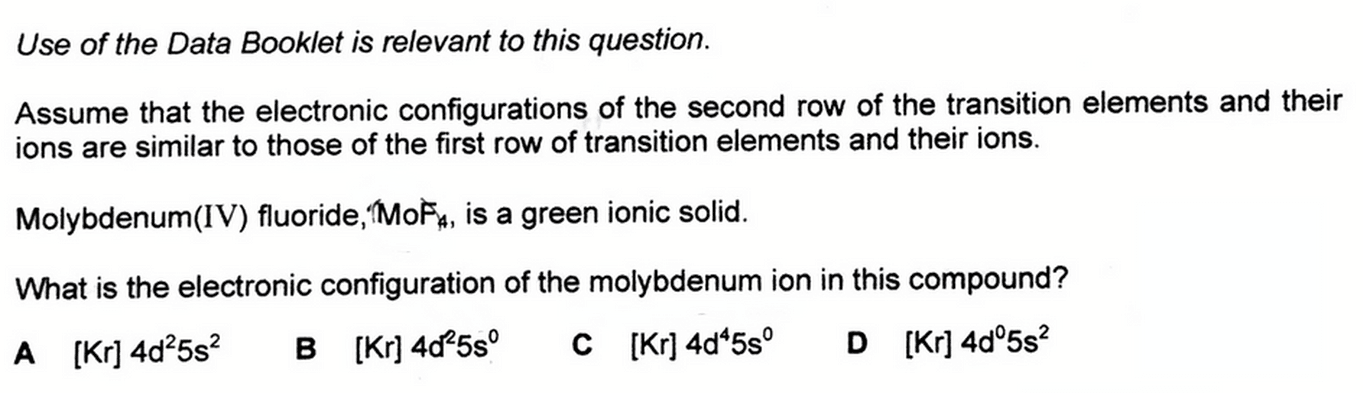
We need to deduce the electronic configuration of molybdenum (IV) ion, given its electronic configuration is similar to those of first row of transition elements and ions.
So the first thing to do is to look up the Periodic Table and find the first row transition element that is directly above molybdenum, which is chromium.
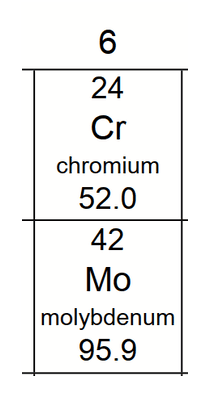
We can figure out the electronic configuration of Cr4+ which is [Ar] 3d2 4s0.
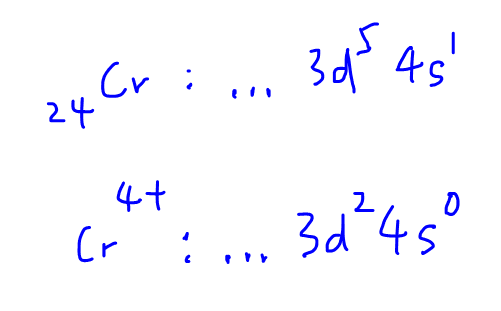
Students might also be interested in knowing how to write out electronic configuration for the first 30 elements.
Therefore the electronic configuration for Mo4+ will be the same as Cr4+ but the principal quantum number increases by 1 since it is in the next Period, ie [Kr] 4d2 5s0.
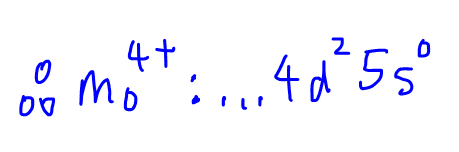
Hence the answer to this question will be option B.
Topic: Transition Elements, Inorganic Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
