Electrophilic Substitution Mechanism of Benzene - Organic Chem
In this video we want to discuss the electrophilic substitution mechanism of benzene using 2 reactions as example.
Nitration of Benzene
The first reaction is nitration of benzene to nitrobenzene.
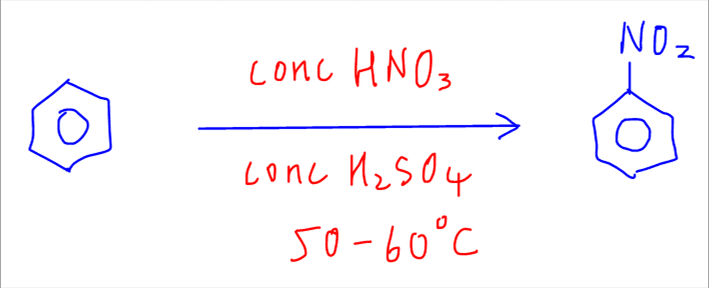
This reaction is done using concentrated nitric acid in concentrated sulphuric acid catalyst at 50 to 60 degree celsius.
The first step involves the generation of electrophile NO2+ which is formed from the reaction between conc HNO3 and conc H2SO4.

In the second step, which is the rate determining step or slow step, benzene will react with the electrophile NO2+.

For arrow pushing we have to draw the full arrow, which represents the movement of 2 electrons, from benzene ring (electron rich region) to nitrogen in NO2+ (electron poor region).
The intermediate formed is highly unstable as the benzene is positively charged and it loses the resonance stability due to the opening of delocalised ring.
In the third step, the intermediate will deprotonate or remove a H+ to form product nitrobenzene. The H+ will combine with HSO4- intermediate to regenerate H2SO4 catalyst.

Bromination of Benzene
The second reaction is bromination of benzene to bromobenzene.
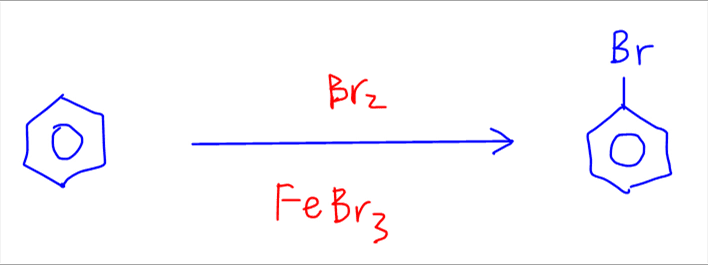
This reaction is done via Br2 in FeBr3 catalyst.
The mechanism is effectively the same, namely:
Step 1 - generate electrophile Br+ from Br2 and FeBr3

Step 2 - carbon in benzene uses 2 electrons from delocalised pi system to form a bond with Br+, generating an unstable intermediate

Step 3 - deprotonation of intermediate to form bromobenzene and regeneration of FeBr3 catalyst.

For the detailed step-by-step discussion on how to draw the mechanism, check out this video!
Topic: Benzene (Hydrocarbon), Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online chemistry classes!
