How to Draw Energy Cycle involving Enthalpy Change of Formation - Energetics
In this video we want to learn how to draw the energy cycle given enthalpy changes of formation of compounds and calculate the enthalpy change of a reaction using Hess' Law.
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through this exercise.
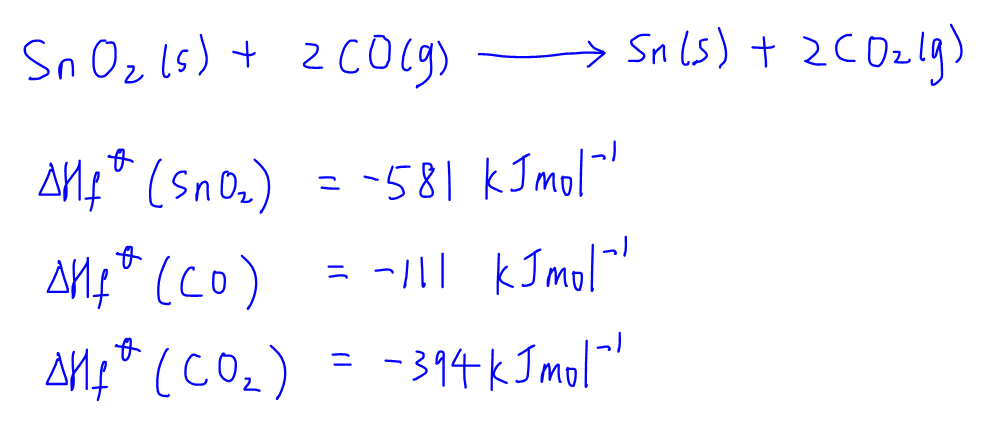
Definition of Enthalpy Change of Formation
In order for us to draw the energy cycle, we need to understand enthalpy change of formation and be able to write out equations to show the enthalpy change of formation of these compounds.
Enthalpy change of formation is defined as the energy change when one mole of compound is formed from its constituent elements in standard states.
For the balanced equation the coefficient of the compound has to be fixed at one since enthalpy change of formation is with respect to per mole of compound:
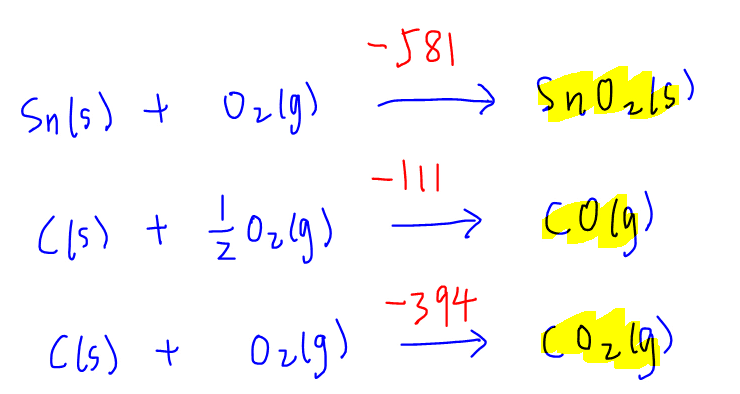
Draw Energy Cycle
Next we can draw the energy cycle by writing out the main equation first:
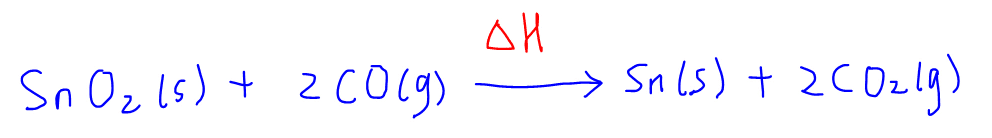
Since we are given the enthalpy change of formation of all the compounds, we can link this equation to elements in the standard state, which we can put below the main equation.
After balancing the elements, the energy cycle will look like this:
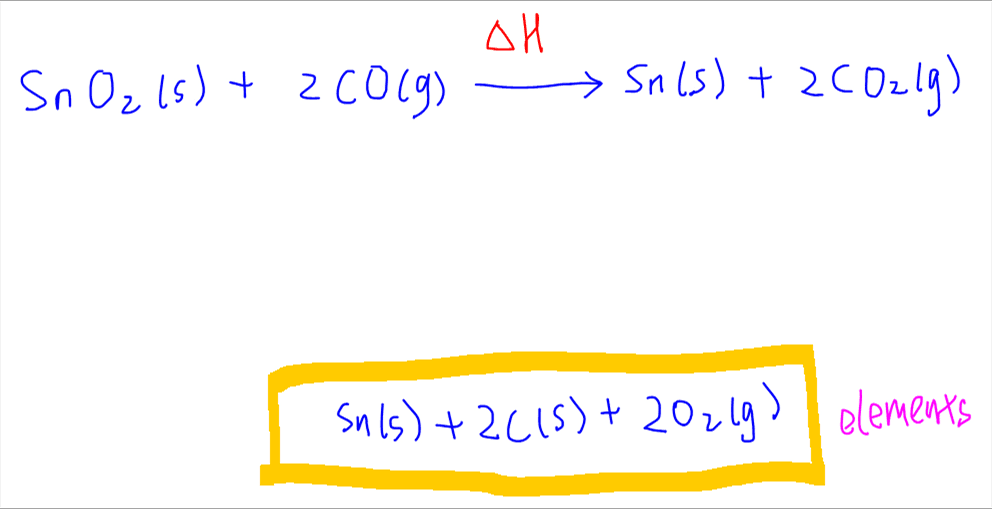
Now we can add arrows for each formation reaction.
First let's account for formation of SnO2. Since coefficient of SnO2 is 1, the enthalpy change of formation for SnO2 is unchanged.
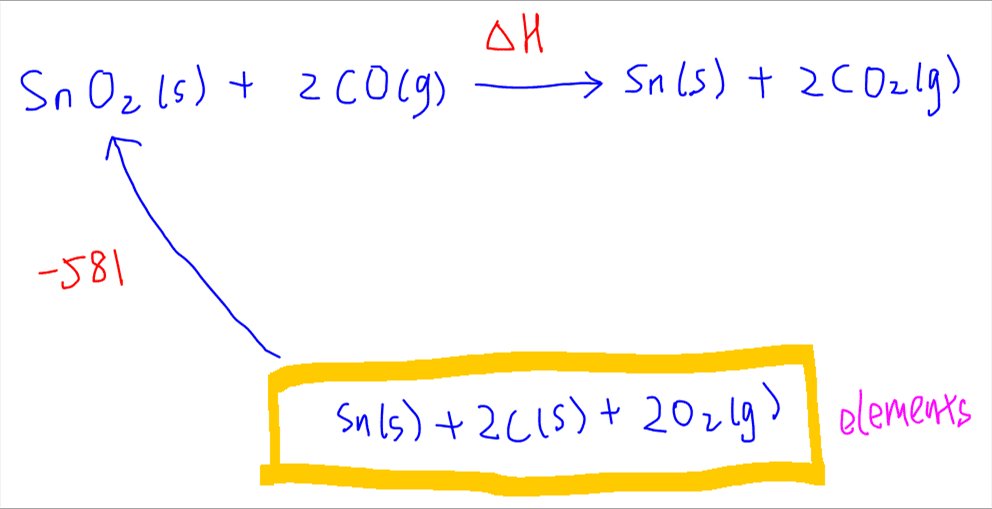
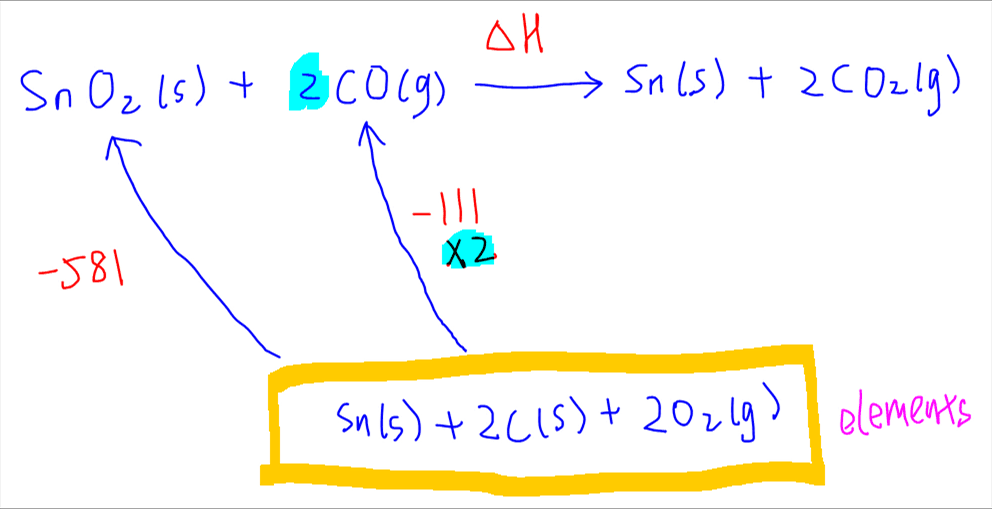
Next for formation of CO. Since coefficient of CO is 2, enthalpy change of formation for CO has to be doubled.
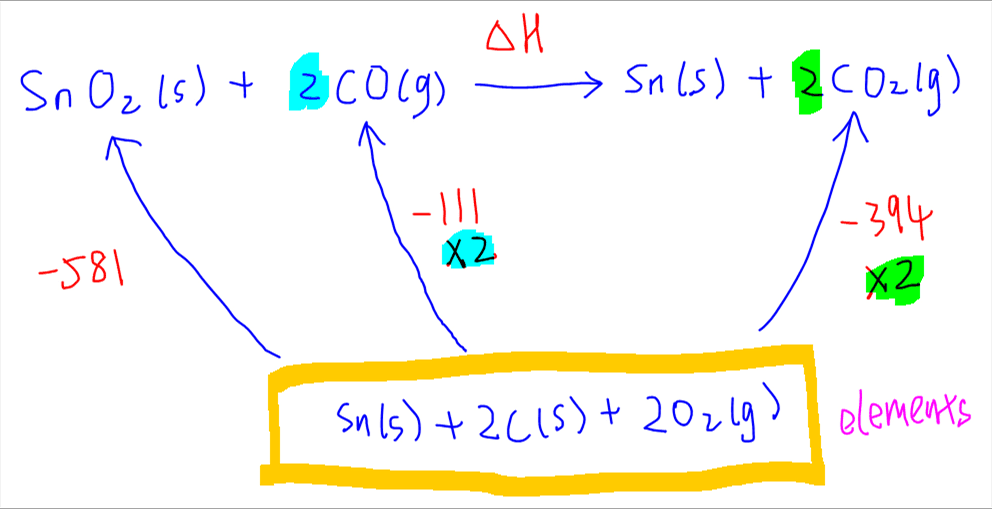
Finally for formation of CO2. Since coefficient of CO2 is 2, enthalpy change of formation for CO2 also has to be doubled.
Apply Hess' Law
Once the energy cycle is complete, we can determine which of these arrows are clockwise and counter-clockwise.
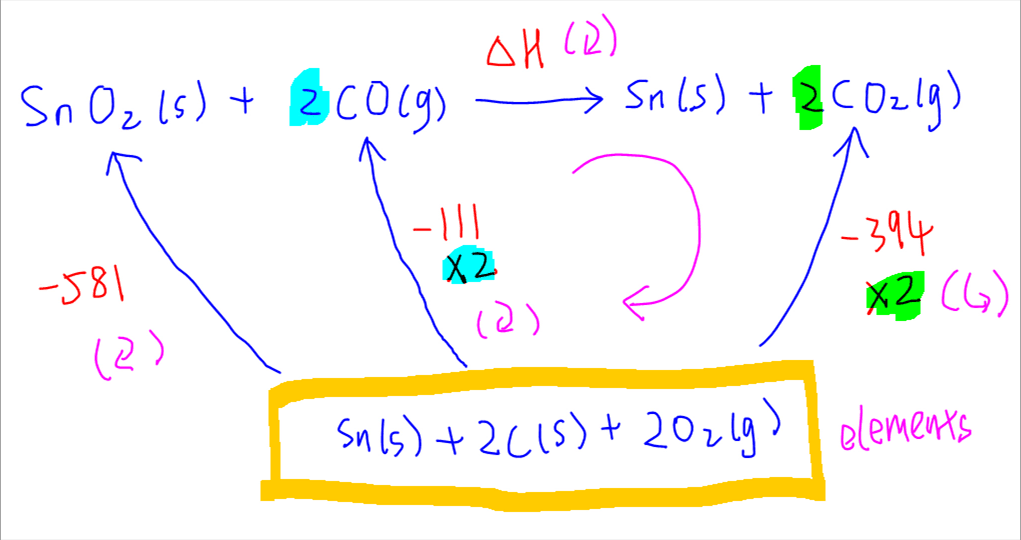
Finally we can apply Hess' Law by putting all the clockwise arrows on one side of the equation and all the counter-clockwise arrows on the other side of the equation to solve for the unknown enthalpy change.
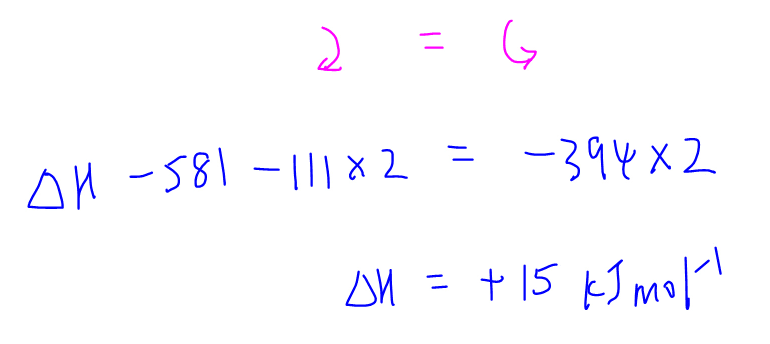
For the detailed step-by-step discussion on how to draw this energy cycle, check out this video!
Topic: Energetics, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's leading JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online chemistry classes!
