Determine Kp given Reactant Partial Pressure
Let Chemistry Guru, Singapore's top choice for JC Chemistry tuition, guide you through this week's question.
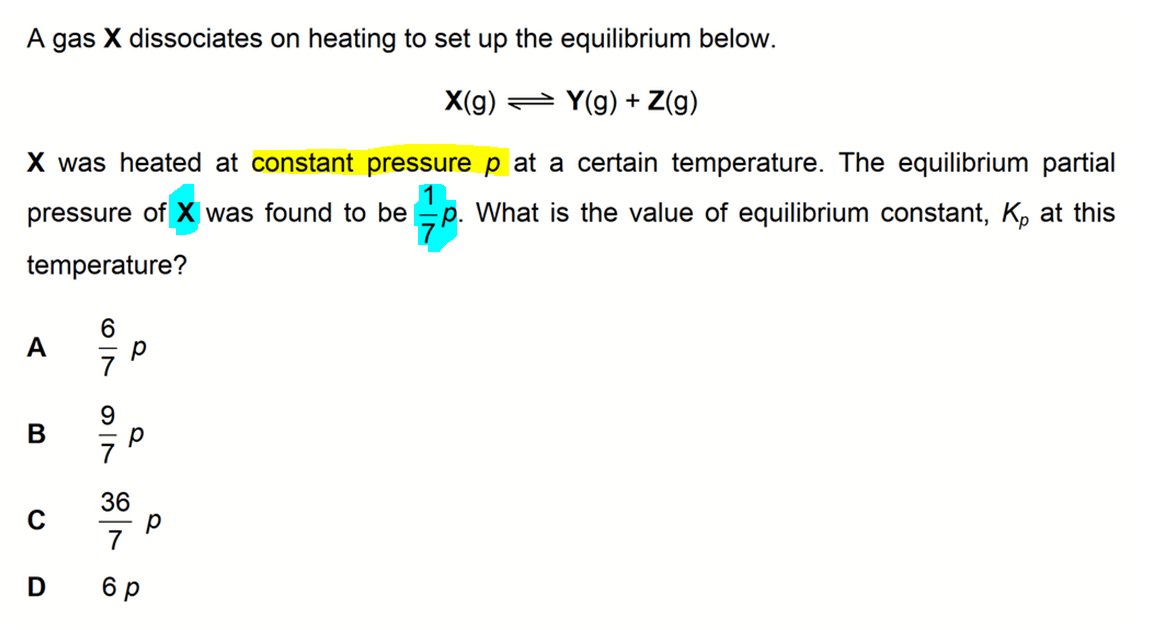
We are required to determine the value of equilibrium constant, Kp, given that the equilibrium partial pressure of reactant X is 1/7 p.
From the balanced equation we can see that the mole ratio for products Y and Z is 1 to 1.
This means the number of moles of Y and Z produced, and their partial pressures at equilibrium will be the same.
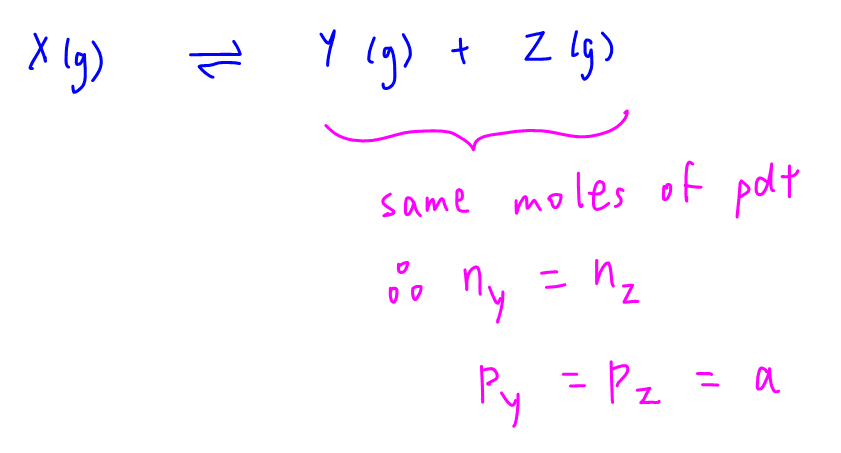
We can add up all the partial pressures of X, Y and Z to find total pressure, which is equal to p since pressure is constant.
Since Px is 1/7 p and Py = Pz, we can determine Py = Pz = 3/7 p
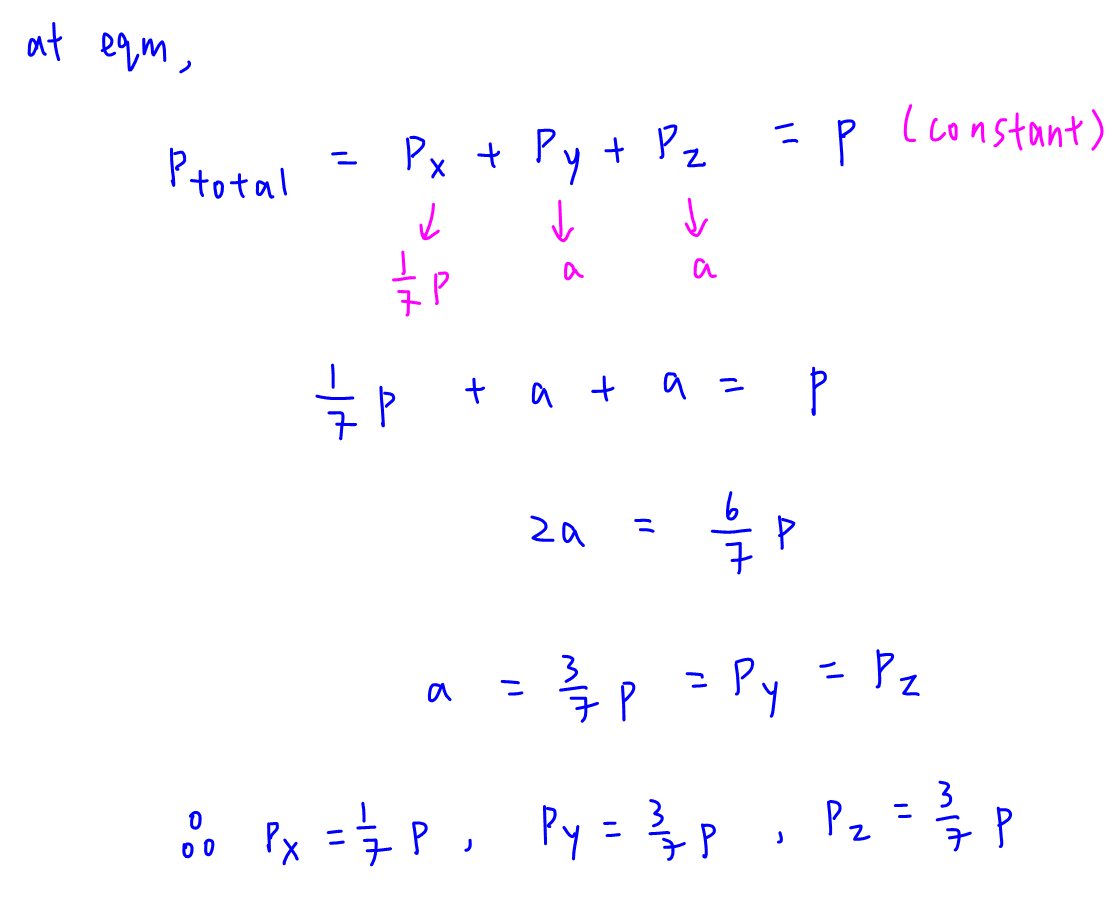
Now that we have determined all the partial pressures at equilibrium, we can write out equilibrium constant Kp and solve for it.
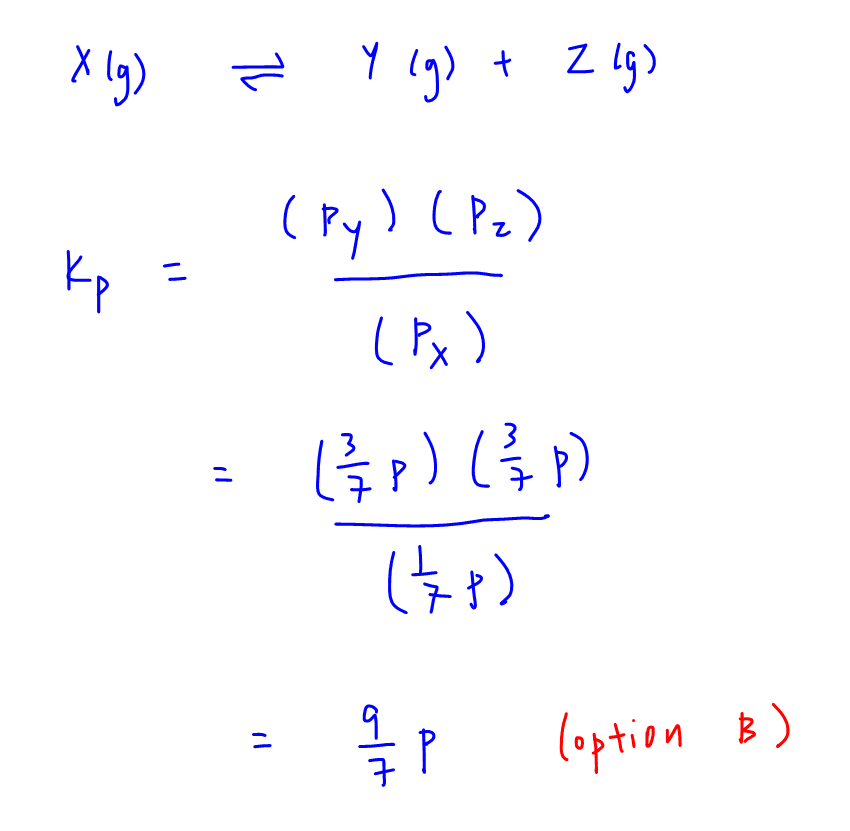
Therefore Kp is calculated to be 9/7 p and option B is our answer.
Topic: Chemical Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's esteemed H2 Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
