First Ionisation Energy Trend Down the Group
Let Chemistry Guru, Singapore's esteemed A Level Chemistry tuition centre, guide you through the first ionisation energies of Group 2 elements.
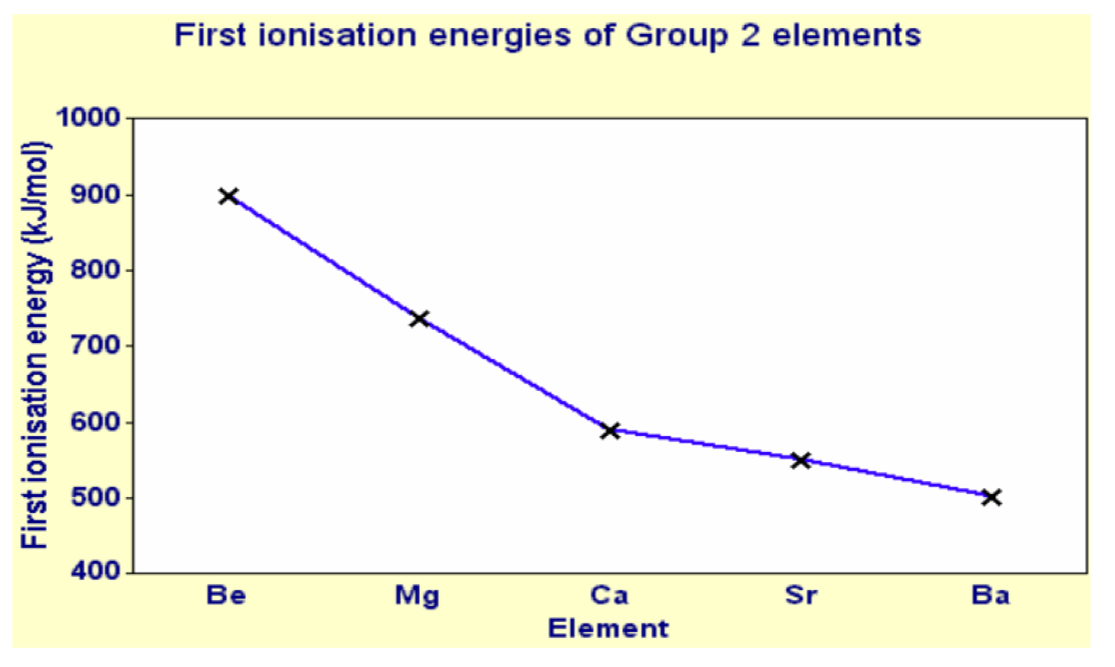
We notice there is a decrease in the first ionisation energy when we go down the Group.
In fact this trend is consistent down any Group.
So why is there a decrease in first ionisation energy down the Group?
We can use effective nuclear charge to explain the trend.
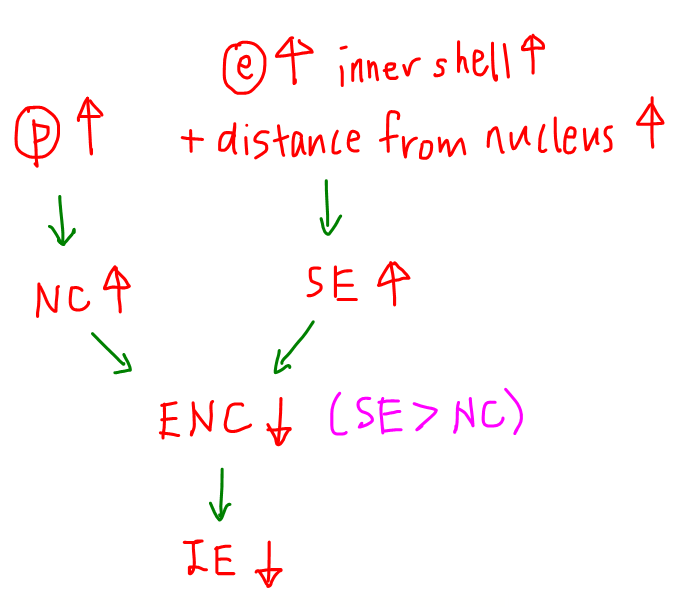
Down the Group, proton number increases so nuclear charge increases.
This will cause a stronger attraction on the valence electrons.
Electrons are added to the next principal quantum shell hence distance from the nucleus increases.
There is also an increase in the number of inner shells so shielding effect increases.
Both the increase in distance from nucleus and shielding effect will cause a weaker attraction on the valence electrons.
Overall the effective nuclear charge decreases and there is an overall weaker attraction on the valence electrons.
Less energy is required to remove the valence electrons hence ionisation energy decreases down the Group.
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
