First Ionisation Energy Trend Across Period 3
In Atomic Structure we need to be familiar with a few ionisation energy trends.
In this video created by Chemistry Guru, Singapore's leading JC Chemistry tuition centre, let's discuss the first ionisation energy trend across Period 3.
First ionisation energy is the energy required to remove 1 mole of electrons from 1 mole of gaseous atoms according to the following equation:
M(g) -> M+(g) + e-
The first ionisation energy (IE) trend across Period 3 is given in the sketch below.
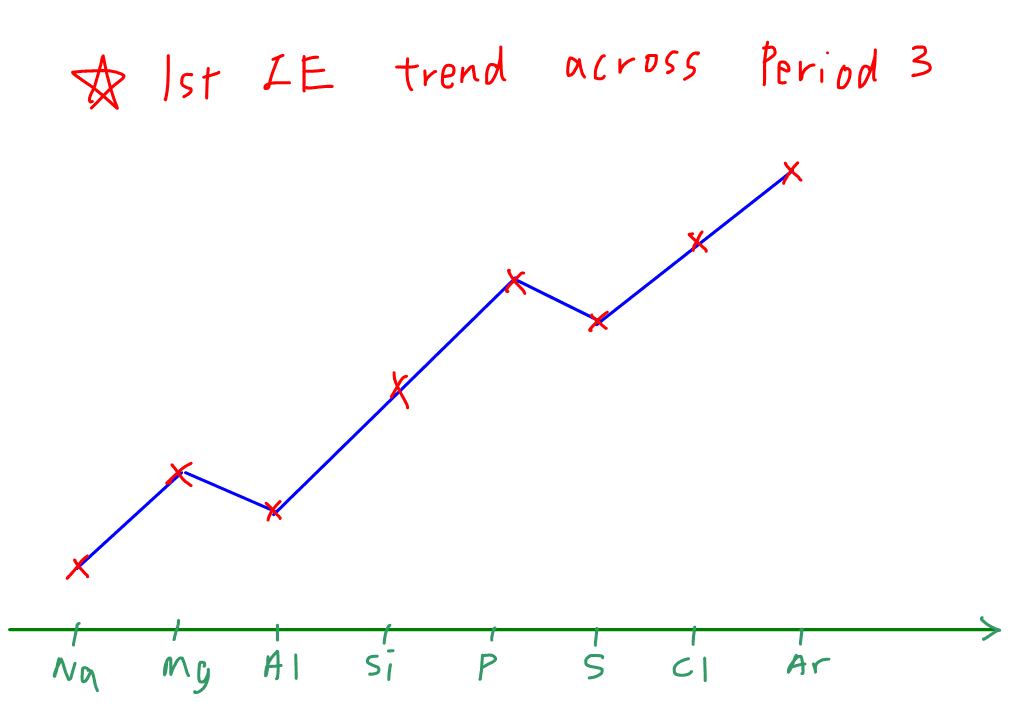
There are 3 observations:
1. General increase in first IE
2. Slight decrease in IE from Mg to Al (first anomaly)
3. Slight decrease in IE from P to S (second anomaly)
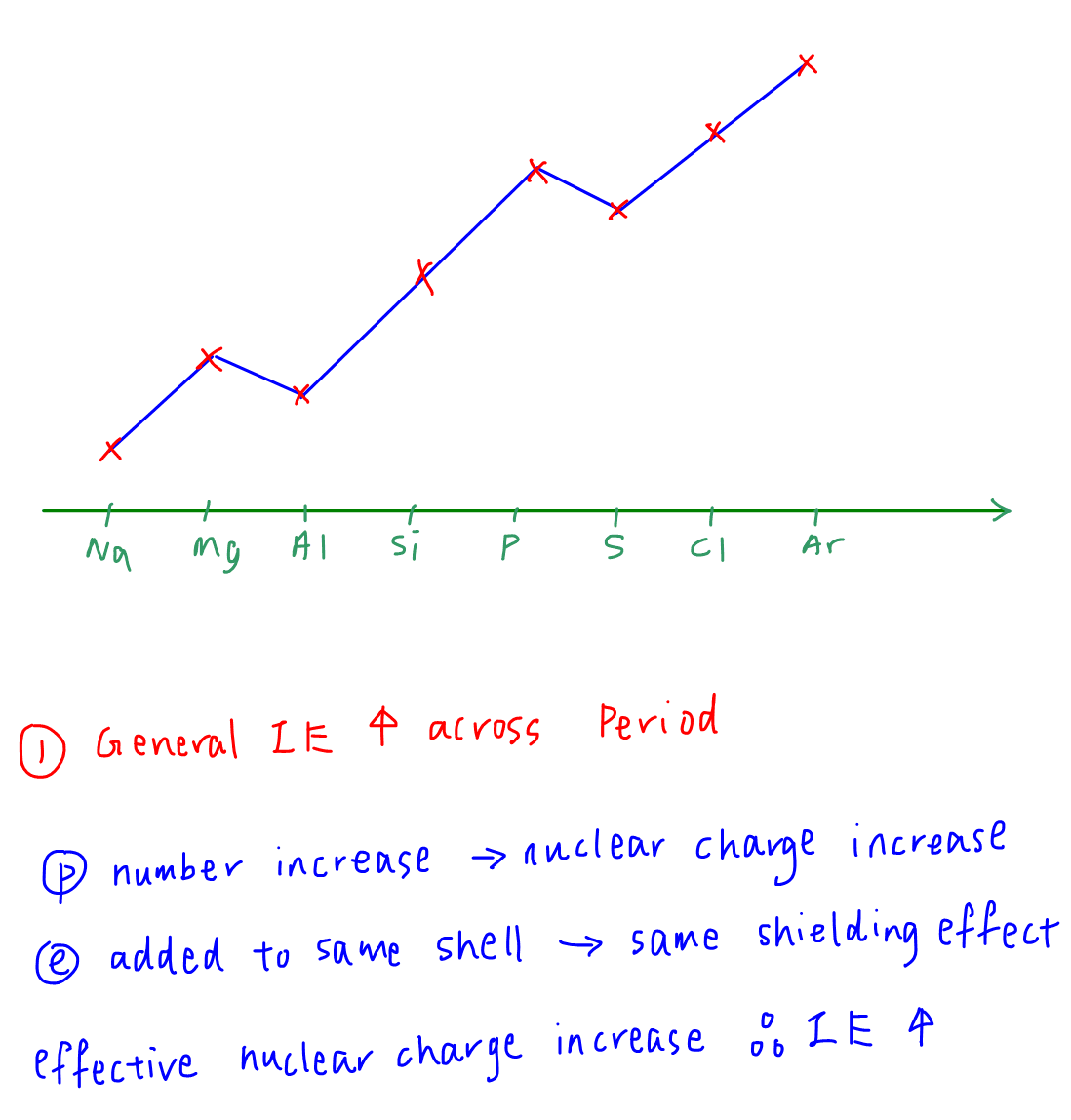
1. General increase in first IE
The concept used to explain the general trend is involving effective nuclear charge.
Across the period proton number increases hence nuclear charge increases.
Electrons are added to the same principal quantum shell so shielding effect or screening effect stays constant.
Effective nuclear charge increases hence attraction between nucleus and valence electron is stronger.
More energy is required to remove the valence electron hence first IE increases across Period 3.
2. Slight decrease in IE from Mg to Al
The first anomaly is the decrease in IE from Mg to Al.
We need to write down the electron configuration for Mg and Al and compare where the electrons are removed.

Electron is removed from 3s subshell for Mg while electron is removed from 3p subshell for Al, which is further away from the nucleus or has a higher energy level.
The attraction between nucleus and that electron is weaker hence less energy is required to remove it.
Therefore Al has a lower first IE.
3. Slight decrease in IE from P to S
The second anomaly between P and S requires us to draw the electron in box diagram as electrons are removed from 3p subshell for both elements.

For sulfur, electron is removed from a fully filled 3p orbital hence it experiences interelectronic repulsion between the electron pair in the same 3p orbital.
This makes it easier to remove that electron, therefore S has a lower first IE.
For the detailed step-by-step discussion on the first ionisation energy trend for Period 3 elements, check out this video!
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
