Free Radical Substitution Mechanism for Alkanes
In this video we want to discuss Free Radical Substitution for Alkanes.
Understanding the free radical substitution mechanism is important for A Level Chemistry, and we need to describe the mechanism in detail using curly arrows.
For this discussion we are using the chlorination of methane.
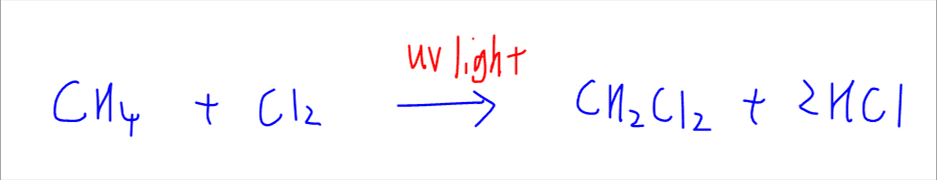
For bromination of methane we simply replace the chlorine with bromine element.
FRS Step 1 - Initiation
The first step of free radical substitution is initiation step, where the chlorine to chlorine bond undergoes homolytic fission to form 2 chlorine atoms or radicals.
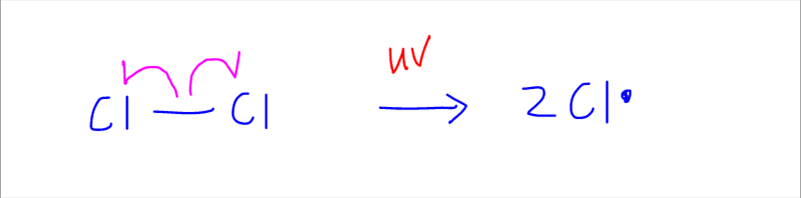
The radicals are extremely reactive and kickstarts the reaction, hence we call this the initiation step.
FRS Step 2 - Propagation
Second step is propagation step, where the radical attacks a stable molecule and generates a stable molecule and another radical.
The total number of radicals stay the same which causes the reaction to propagate, hence the name propagation.
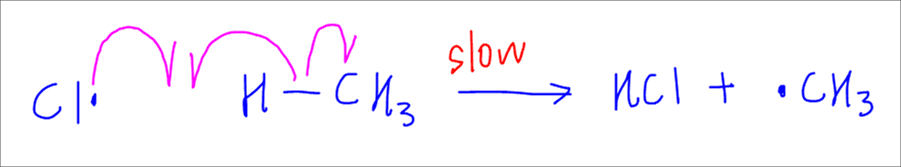
First propagation step is where the chlorine radical attacks methane, breaks the C-H bond homolytically and forms HCl to generate a methyl radical.
Second propagation step is where the methyl radical attacks Cl2 molecule, breaks the Cl-Cl bond homolytically and forms CH3Cl or chloromethane and generate a chlorine radical.
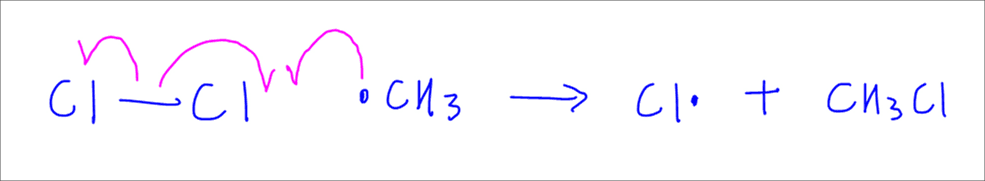
Take note 2 propagation steps are required for one substitution.
So for this example, since we are doing a disubstitution, we need 2 more propagation steps for the second substitution to form dichloromethane, CH2Cl2.
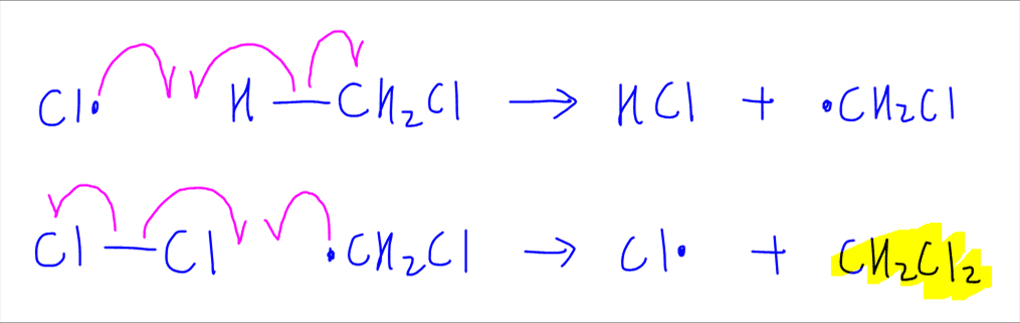
Also, it is important to note that free radical substitution is a totally random process, so the mechanism that we are describing is not the only steps that are taking place, but the shortest pathway to reach the desired product that is requested in the question.
Once we form the required product, CH2Cl2, we can move on to the termination step.
FRS Step 3 - Termination
Finally, last step will be termination step where any two radicals will undergo homolytic fusion and form a stable product.
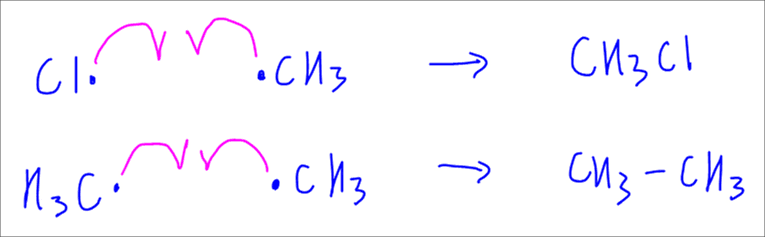
An interesting product from termination step is the formation of ethane, where the carbon number is double that of starting alkane, methane in this case.
For the detailed discussion on how to draw the mechanism, check out this video!
Topic: Alkanes (Hydrocarbon), Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
A Level H2 Chemistry Video Lessons
Chemistry Guru, Singapore's reputable JC Chemistry tuition centre, has a huge collection of short video lessons that targets important H2 Chemistry concepts and common questions.
Join my 19,000 subscribers on my YouTube Channel for new video lessons every week!
Follow me on Instagram for H2 Chemistry videos and (not so funny) memes!
You can also view other A Level H2 Chemistry videos here at my website.
Need an experienced tutor to make Chemistry simpler for you?
My weekly classes in Singapore are ideal for students who prefer a more structured program.
Build a strong foundation and ace your exams!
Sign up now for a trial lesson at $50only!
Check out our Chemistry tuition class timing and topics covered for our popular JC1 Classes and JC2 Classes at Bishan, Singapore.
Online lessons are also available! Learn H2 Chemistry anytime, anywhere at 50% of the cost of conventional class tuition.
Find out more information about our online tuition.
