Friedel Crafts Alkylation
Let Chemistry Guru, Singapore's esteemed A Level Chemistry tuition centre, guide you through our discussion question this week!
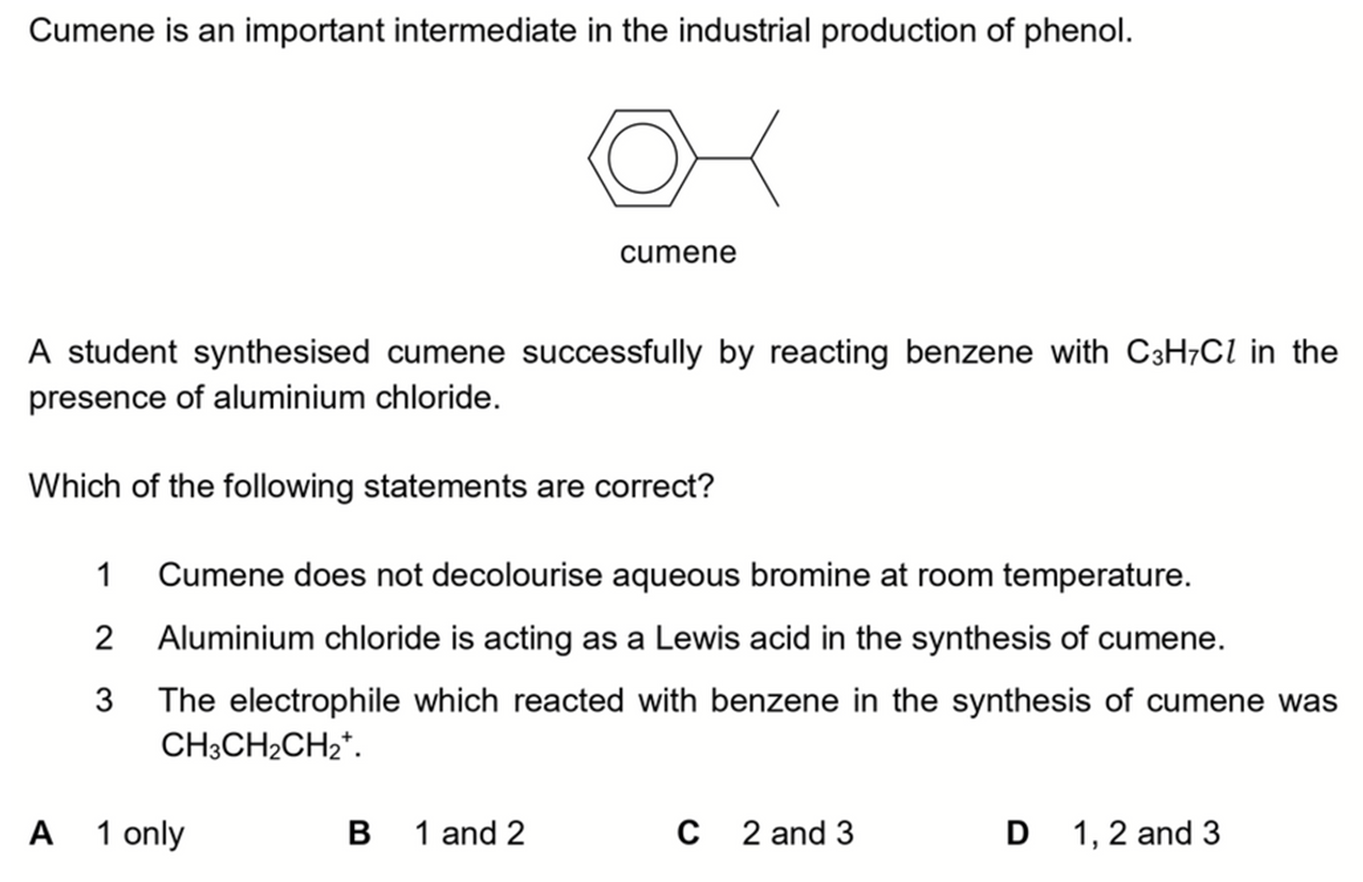
We want to determine which of the statements regarding Friedel Crafts alkylation of benzene are correct.
From product cumene we can deduce the reactant is 2-chloropropane, with AlCl3 as catalyst.

1. Cumene does not decolourise aqueous bromine at room temperature.
Alkyl groups are activating and make benzene slightly more reactive, but bromination will still require presence of catalyst such as FeBr3.
Only highly activating groups like -OH and -NH2 groups will make benzene reactive enough to react with bromine without the need for catalyst.
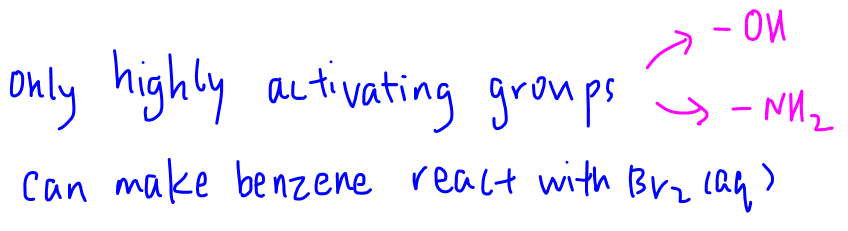
Hence statement 1 is true.
2. Aluminium chloride is acting as a Lewis acid in the synthesis of cumene.
Let Chemistry Guru, Singapore's esteemed A Level Chemistry tuition centre, guide you through the electrophilic substitution mechanism of benzene to form cumene.
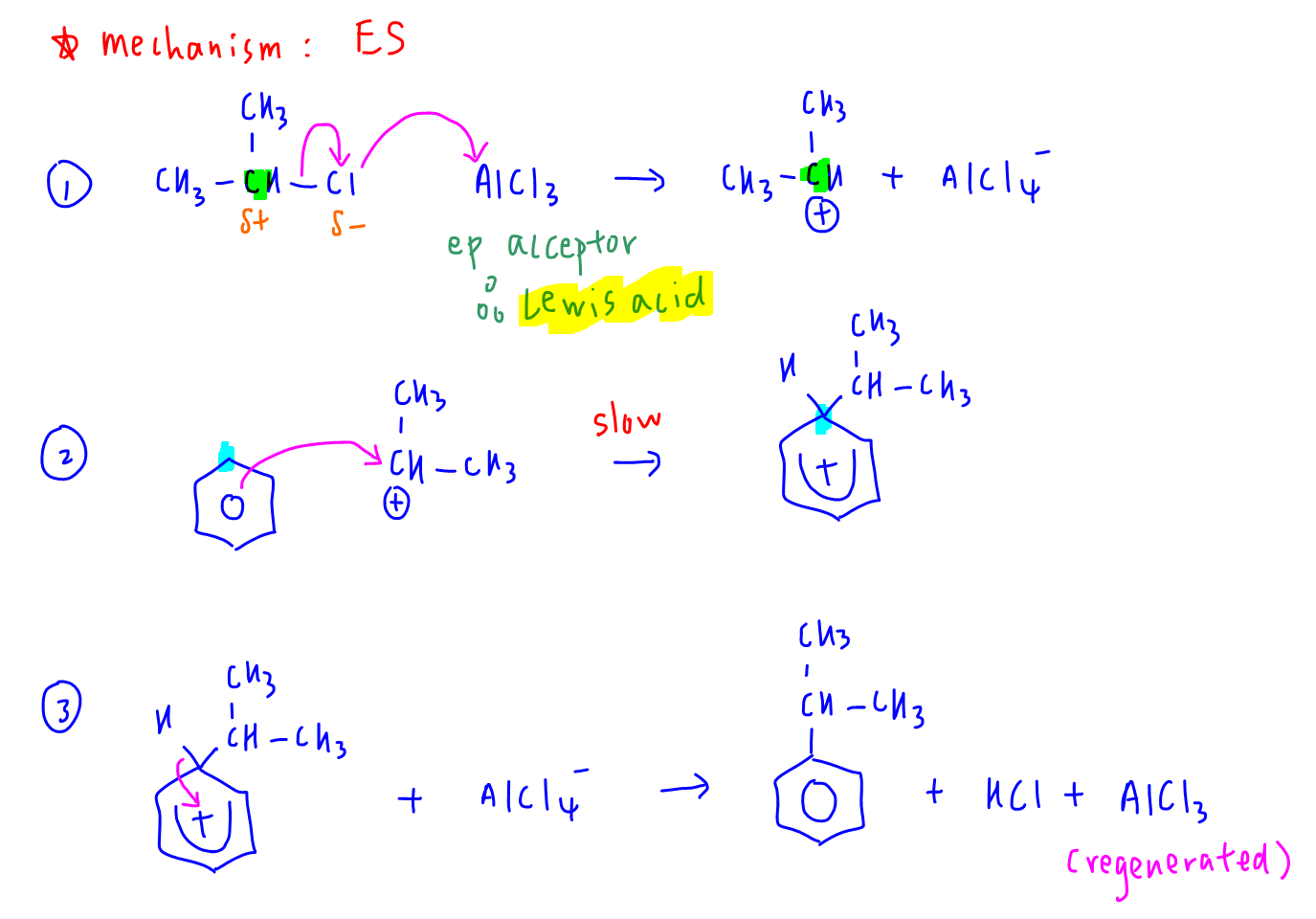
In step 1, AlCl3 acts as an electron pair acceptor from Cl- to generate the electrophile.
Hence statement 2 is true.
3. The electrophile which reacted with benzene in the synthesis of cumene was CH3CH2CH2+.
Since reactant is 2-chloropropane, the electrophile should have positive charge on second carbon instead of terminal carbon.

Hence statement 3 is false.
Therefore the answer to this question is option B.

Topic: Benzene, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
