High Spin and Low Spin Complexes
Transition metal complexes can exist as high spin or low spin depending on the strength of the ligands.
Let's understand how the strength of ligands affect the spin of the complex.
This concept involving high spin and low spin complexes is not in A Level Chemistry syllabus but has appeared in some Prelim questions.
1. Gaseous Fe(III) cation
The electronic configuration for Fe3+ is given as 1s2 2s2 2p6 3s2 3p6 3d5
We can also determine the electron in box diagram for 3d subshell.

Notice there are 5 unpaired electrons in 3d subshell for Fe3+.
2. Hexaaquairon(III) complex
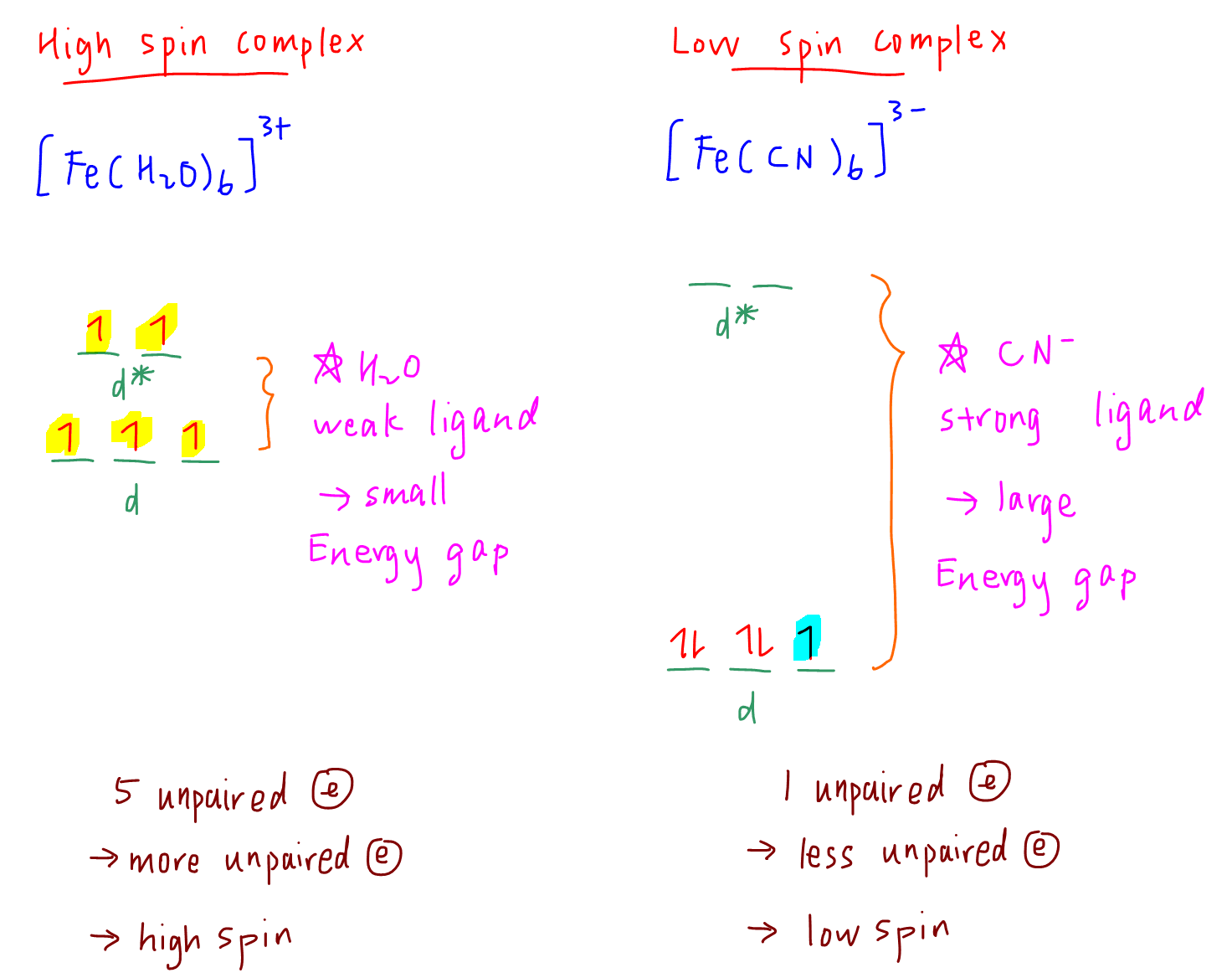
Since oxidation state of iron is still +3, there are still 5 electrons in 3d subshell in [Fe(H2O)6]3+ complex.
In a complex the ligands will interact with the d orbitals to different extent depending on the shape of the complex.
Therefore the d orbitals that interact more with the ligands will have a higher d* energy level, while the d orbitals that interact less will have a lower d energy level.
This is known as d-d* orbital splitting.
For octahedral complexes, the splitting pattern is 2 orbitals at higher d* level and 3 orbitals at lower d level.
Water is a weak ligand and the energy gap between d to d* level is small.
Hence the d electrons will ignore the small energy difference and be filled in the same way as in gaseous Fe3+ cation, where electrons will occupy orbitals singly and with parallel spins.

Notice there are 5 unpaired electrons, hence hexaaquairon(III) complex is considered a high spin complex.
3. Hexacyanoferrate(III) complex
CN- is a strong ligand and will cause the energy gap between d to d* level to be larger.
It requires too much energy to put the d electrons at the higher d* level, so electrons will pair up at the lower d level first.

Notice there is now only 1 unpaired electron, hence hexacyanoferrate(III) complex is considered a low spin complex.
Comparing both high spin and low spin complexes:
- a weak ligand such as H2O will cause a smaller d-d* energy gap and tend to form high spin complexes
- a strong ligand such as CN- will cause a larger d-d* energy gap and tend to form low spin complexes
Topic: Transition Elements, Inorganic Chemistry, A Level Chemistry, Singapore
Back to other previous Inorganic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
