How Concentration Affects Electrolysis Product
When the electrolyte is concentrated, the products of electrolysis can be affected.
This applies to concentrated ions with small electrode potential difference between the species to be discharged at the electrode.
Let's use the electrolysis of brine or concentrated NaCl as an example.
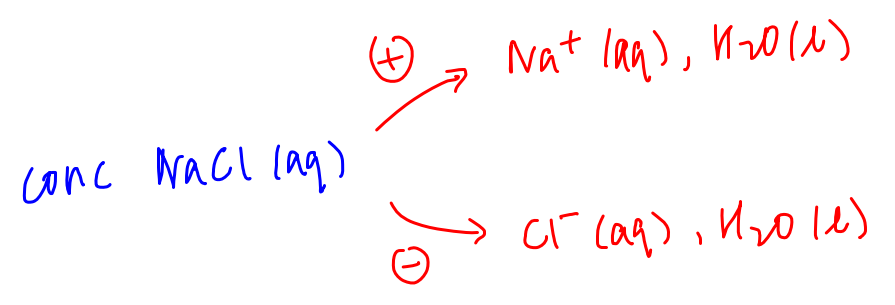
1. Reduction at Cathode
Species migrating to cathode will be Na+ and water.
Since reduction occurs at the cathode, we need to find both species on the left hand side of half equation in the Data Booklet.
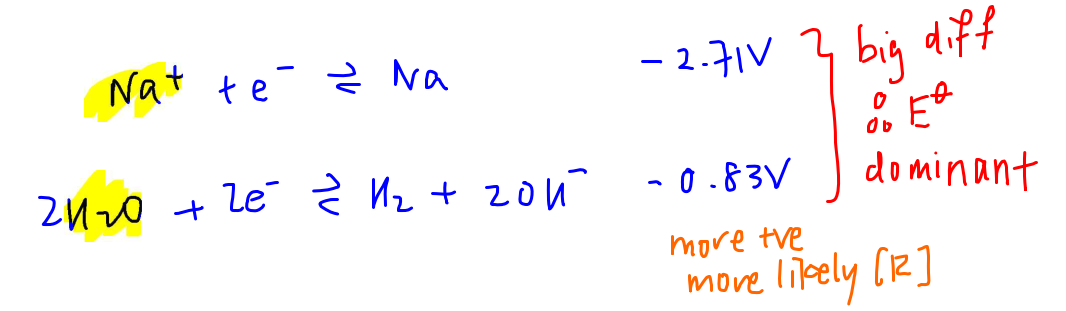
The E value for Na+ is much more negative than E value for H2O.
This means it's much harder to reduce Na+ as compared to water, even if Na+ is concentrated.
Hence water will be reduced at the cathode.
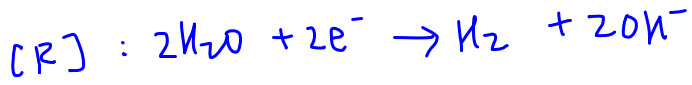
2. Oxidation at Anode
Species migrating to anode are Cl- and water.
We need to find both species on the right hand side of half equation since oxidation occurs at the anode.
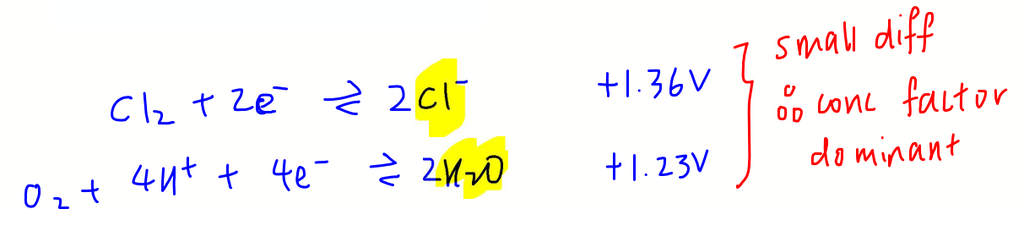
The E value for Cl- is very close to E value for H2O.
This means the ease of oxidation for both species are similar, E value factor is less significant and concentration is the dominant factor.
Since Cl- is concentrated, it will be oxidised at the anode.

Topic: Electrochemistry, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
