Deduce How Rate Constant and Equilibrium Constant Change with Temperature

1. Deduce Rate Constant k
Rate constant k is affected by temperature and activation energy.
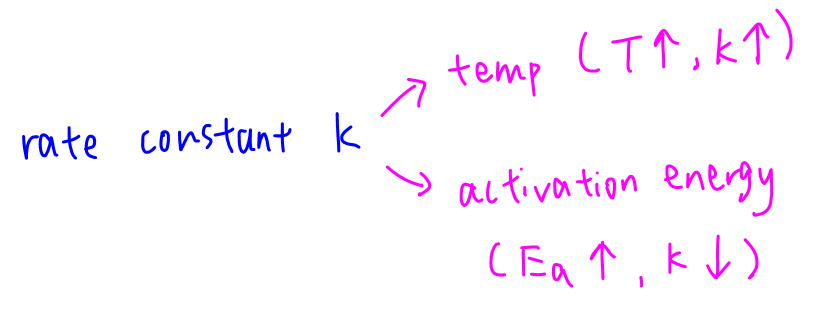
a. When temperature increases, average kinetic energy of reactant particles increases.
There are more particles with energy greater than activation energy, frequency of successful collision increases, hence rate and rate constant increase.
Therefore both k1 and k-1 will increase when temperature increases.
b. When activation energy increases, there are less particles with energy greater than activation energy, frequency of successful collision decreases hence rate and rate constant decrease.
2. Deduce Equilibrium Constant Kc
When temperature increases, position of equilibrium shifts left to favour endothermic reaction in order to absorb excess heat.
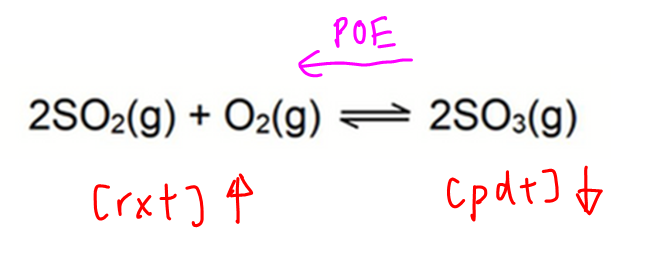
Reactant concentration increases while product concentration decreases.
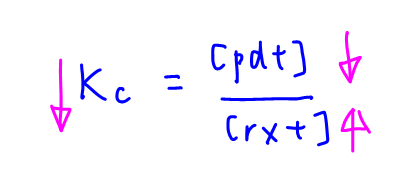
Therefore Kc decreases when temperature increases.
Hence answer to this question is option D.

Topic: Chemical Equilibria, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's prestigious JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
