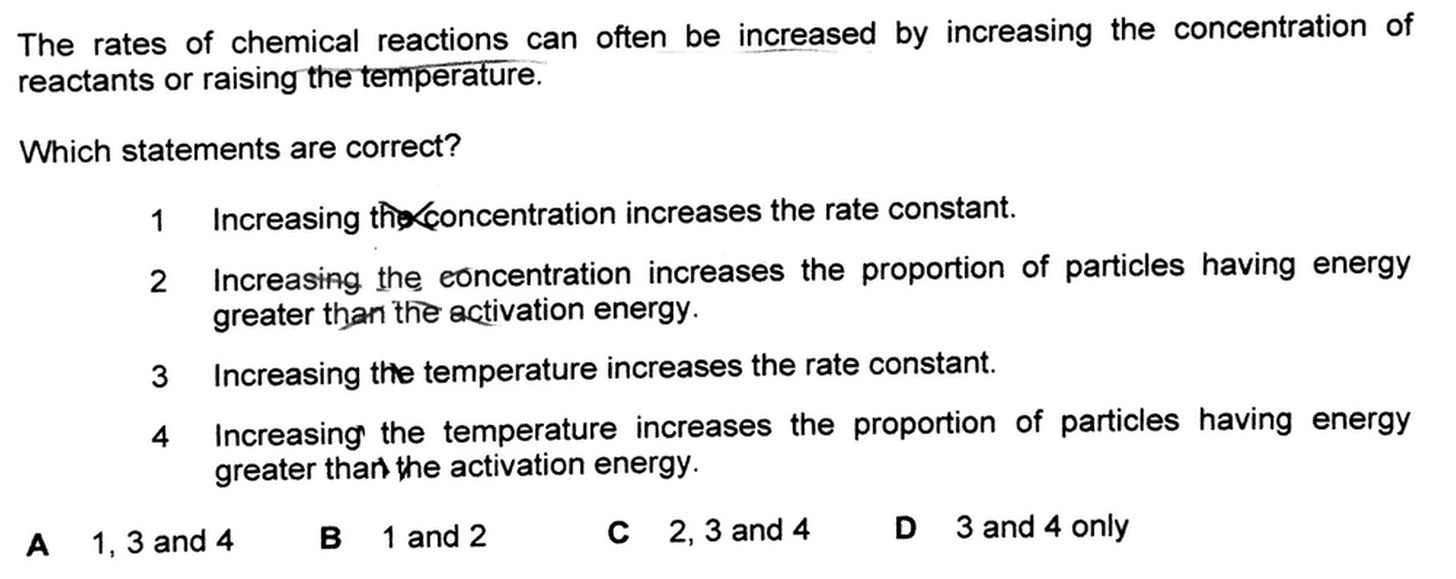
2019 A Level H2 Chemistry Paper 1 Question 15 - How Temperature and Reactant Concentration Affect Rate
2019 A Level H2 Chemistry Paper 1 Question 15 is related to kinetics. Let's take a look.
First let's recap the rate equation and what factors affect the rate of a reaction.

Rate of a reaction is affected by rate constant k and the concentration of reactants.
m represents the order of reaction with respect to reactant, and can be either 0, 1 or 2 in A Level Chemistry Syllabus.
Rate constant k in itself is affected by 2 terms: temperature and activation energy.
Higher temperature will cause a bigger rate constant k hence rate increases.
Lower activation energy (via the use of catalyst) will cause a bigger k hence rate increases.
Therefore the rate of the reaction can be increased by:
- increasing temperature (affects k)
- add catalyst to lower activation energy (affects k)
- increase concentration of reactant
We can now run through the statements and see if they are correct.
1. Increasing the concentration increases the rate constant
Rate constant is affected by temperature and activation energy only.
Increase concentration will affect rate but not rate constant.

Hence statement 1 is not correct.
2. Increasing the concentration increases the proportion of particles having energy greater than the activation energy
Increasing the proportion of particles having higher kinetic energy is only done via increasing temperature.
Temperature is the measure of average kinetic energy of particles in a system.
Higher temperature means higher kinetic energy and vice versa.
This means changing concentration will have no effect on kinetic energy.

Hence statement 2 is also not correct.
3. Increasing the temperature increases the rate constant
This is true since rate constant is affected by temperature.
Higher temperature means greater rate constant.
Hence statement 3 is correct.
4. Increasing the temperature increases the proportion of particles having energy greater than the activation energy
Higher temperature means greater kinetic energy for reactant particles.
Hence there will be more particles with energy greater than activation energy.
Statement 4 is correct.
Finally we can compare the options, keeping in mind only statements 3 and 4 are correct.
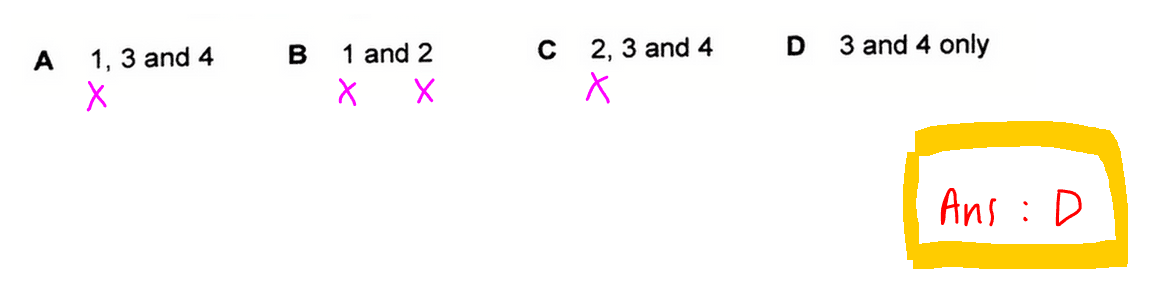
Hence we can conclude the answer to this question is option D.
Topic: Kinetics, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan, weekly LIVE webinars or online tuition classes!
