Hybridisation of Carbon in Ethene
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through the question.
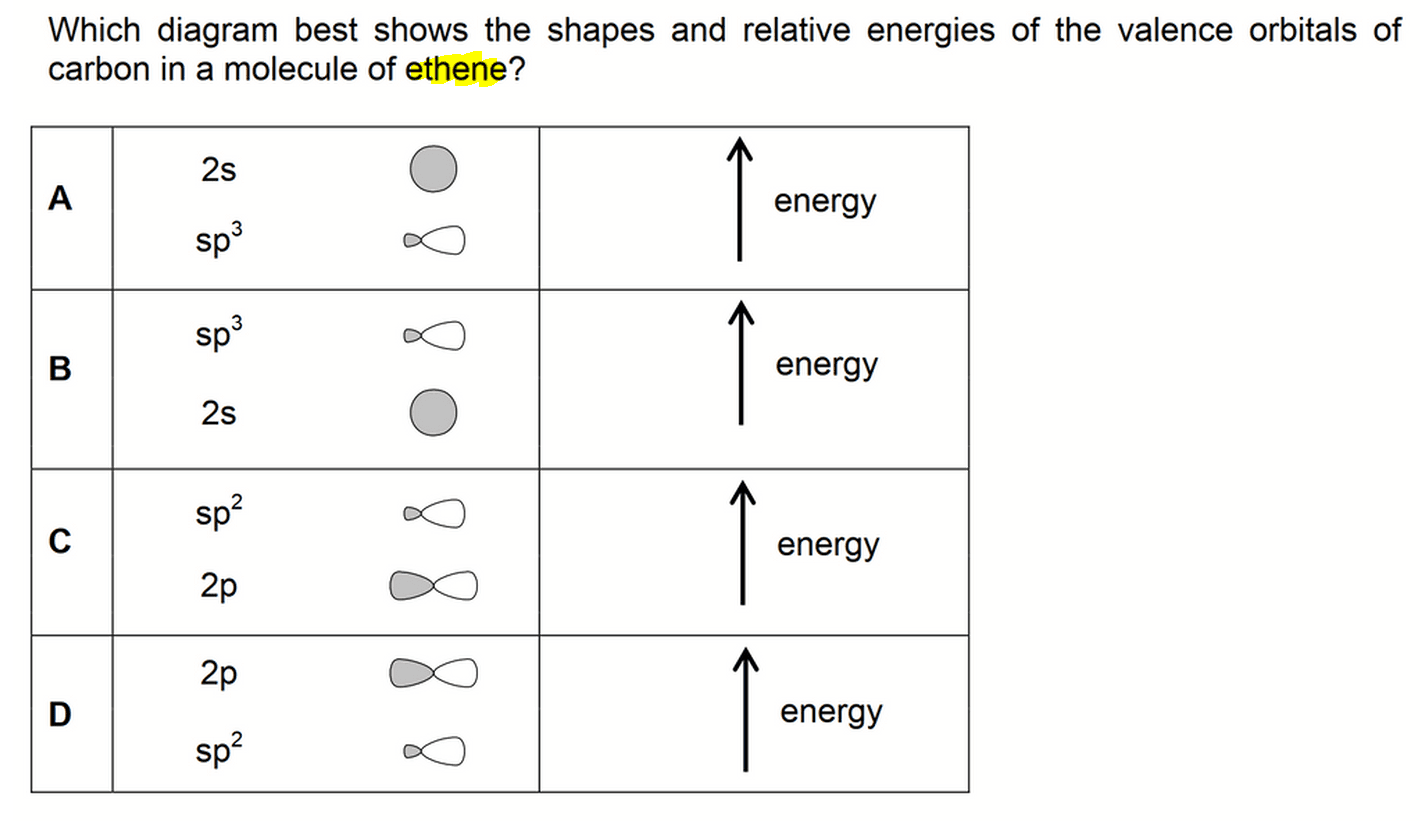
We need to deduce the valence orbitals in ethene and compare their relative stability.
If we consider carbon in the ground state, its electronic configuration will be 1s2 2s2 2p2.
There are 4 valence electrons but only 2 electrons are unpaired in 2p subshell.
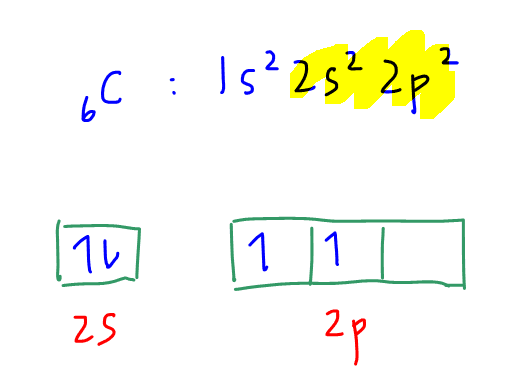
This means that carbon in the ground state can only form 2 bonds since it has only 2 unpaired electrons.
So carbon will undergo excitation where an electron in 2s subshell is promoted to 2p subshell.
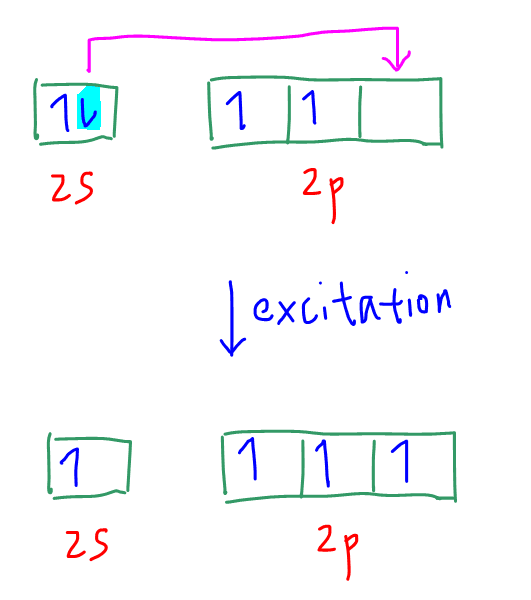
Now we have 4 unpaired electrons and carbon can form 4 bonds.
If carbon uses 2s orbital (spherical) and 2p orbitals (dumb-bell shaped) to form bonds directly, the overall shape of carbon in ethene will not be trigonal planar.
Hence we need the concept of hybridisation to explain its shape.
I have a video lesson discussing hybridisation of carbon in detail so do check it out if you are keen.
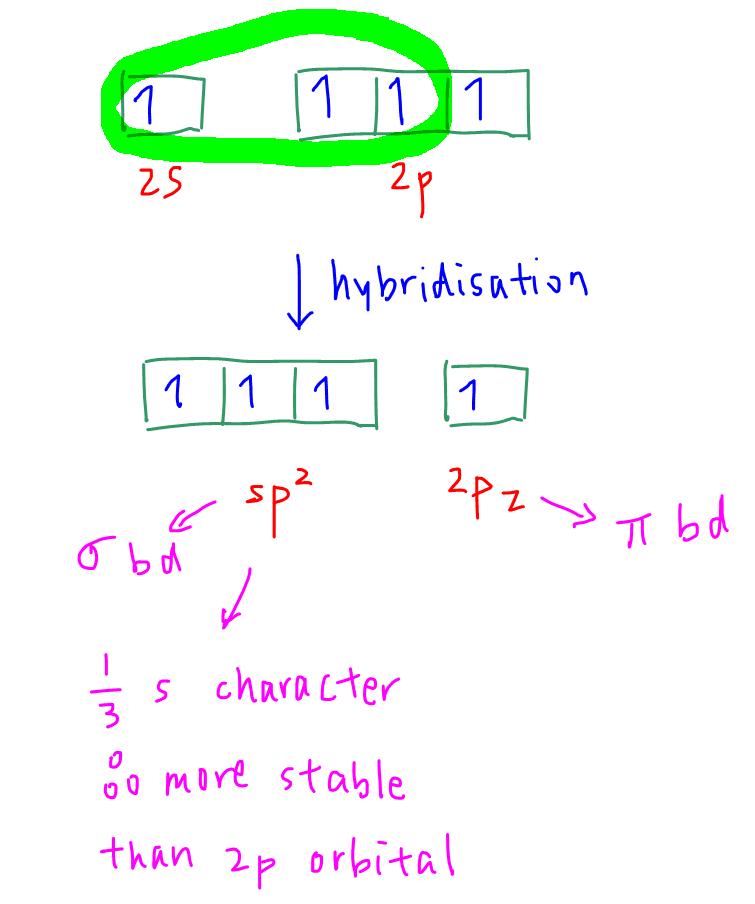
Hybridisation is the mixing of valence orbitals to form sigma bonds.
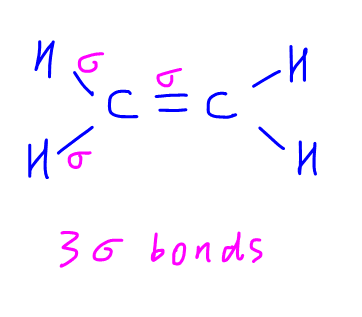
Carbon in ethene has 3 sigma bonds.
Hence carbon will mix 3 valence orbitals (2s, 2px and 2py) to form 3 sp2 hybridised orbitals that are equal in shape and energy.
Each sp2 hybridised orbital will be involved in sigma bond formation.
Arrangement of the hybridised orbitals with respect to carbon is trigonal planar.
2pz is not hybridised and will be involved in pi bond formation.
Therefore the valence orbitals for carbon in ethene will be sp2 and 2pz orbitals. (option A and B eliminated)
Comparing energy level, sp2 orbitals have 1/3 s character hence will be more stable than 2p orbitals.
Therefore sp2 orbital will have lower energy than 2p orbital. (option C eliminated)
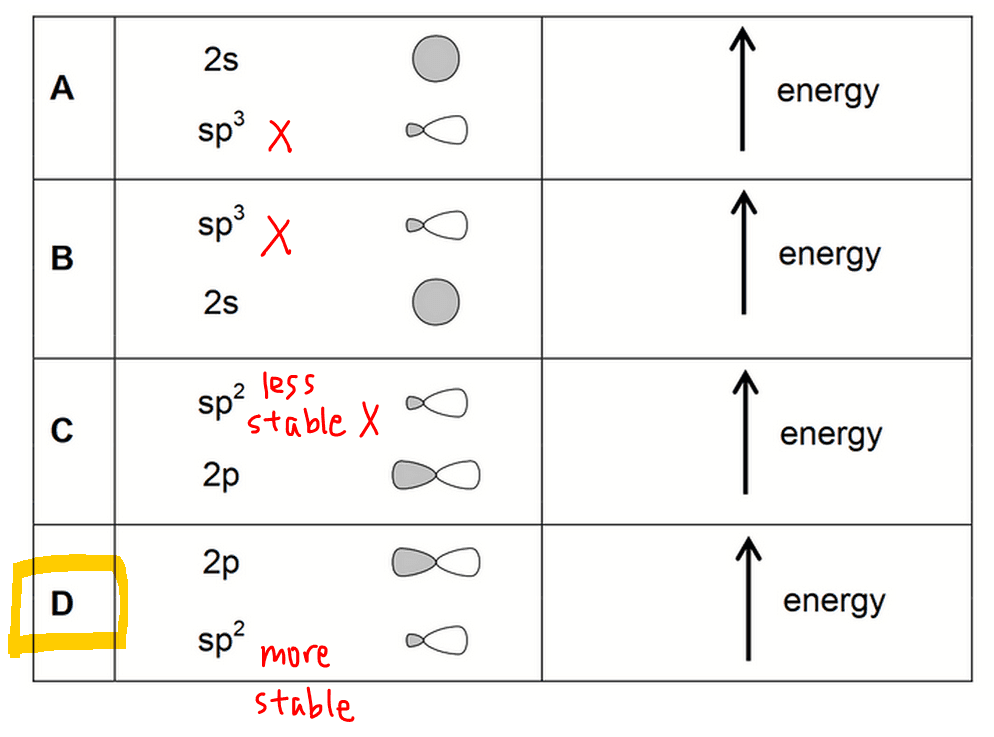
Finally we can determine the answer to this question is option D.
Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
