Hybridisation of Carbon in Organic Compounds
In this video we want to discuss the concept of excitation and hybridisation of Carbon in Organic Compounds.
We can use hybridisation to explain the shape of organic molecules with respect to carbon.
1. Methane, CH4
Let's consider carbon in methane, CH4.
We know that the shape with respect to carbon is tetrahedral according to Valence Shell Electron Pair Repulsion Theory (VSEPR).
Let's try to explain the shape via the electronic configuration of carbon instead.
Carbon in the ground state has the following electronic configuration: 1s2 2s2 2p2.

Carbon has only 2 unpaired electrons in this case, so therefore it can only form 2 bonds in its ground state.
Of course we know that carbon always forms 4 bonds, so the concept of excitation comes in where an electron is promoted from the paired 2s orbital which is lower in energy to an empty 2p orbital which is higher in energy.

Carbon in its excited state now has 4 unpaired electrons so can form 4 bonds.
However, if carbon just uses these 2s and 2p orbitals for bond formation, the shape with respect to carbon will not be tetrahedral since 2s orbital is more stable and closer to the nucleus so bond formed will be shorter, while 2p orbital is less stable and further away from the nucleus so bond formed will be longer.
This means we will have one shorter C-H bond (from 2s orbital) and 3 longer C-H bonds (from the 2p orbitals), which is hardly the highly symmetrical tetrahedral shape that we see in methane.
Therefore we need another concept to connect between excitation and the final shape of methane - hybridisation.
Hybridisation is the mixing of valence orbitals to form sigma bonds.
Since carbon in methane forms 4 sigma bonds, it will mix 4 of its valence orbitals (2s, 2px, 2py, 2pz) to form 4 identical orbitals with equal shape and energy.

The name of the hybridised orbitals will be sp3 hybridised orbitals and since they have the same shape and energy, they repel each other equally and give sp3 hybridised carbon in CH4 its highly symmetrical tetrahedral shape.
2. Ethene, C2H4
Carbon in ethene forms 3 sigma bonds and 1 pi bond.

Since carbon forms 3 sigma bonds, it will mix 3 of its valence orbitals (2s, 2px, 2py) to form 3 identical orbitals with equal shape and energy.

The name of the hybridised orbitals will be sp2 hybridised orbitals and since they have the same shape and energy, they repel each other equally and give sp2 hybridised carbon in C2H4 its trigonal planar shape.
Remainder 2pz orbital is unhybridised and is used in pi bond formation.
3. Ethyne, C2H2
Carbon in ethyne forms 2 sigma bonds and 2 pi bonds.

Since carbon forms 2 sigma bonds, it will mix 2 of its valence orbitals (2s, 2px) to form 2 identical orbitals with equal shape and energy.

The name of the hybridised orbitals will be sp hybridised orbitals and since they have the same shape and energy, they repel each other equally and give sp hybridised carbon in C2H2 its linear shape.
Remainder 2py and 2pz orbitals are unhybridised and used in pi bond formation.
4. Summary for State of Hybridisation for Carbon
The following table will summarise what we need to know for hybridisation. Based on the number of sigma bonds that carbon forms, we can determine its state of hybridisation.
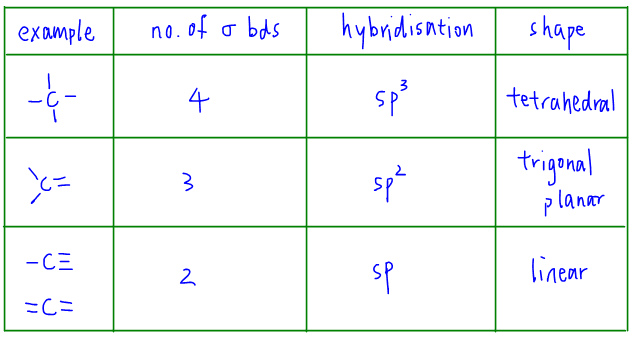
For the detailed discussion and explanation on excitation and hybridisation, check out this video now!
Topic: Introduction to Organic Chemistry, Organic Chemistry, A Level Chemistry, Singapore
Back to other previous Organic Chemistry Video Lessons.
A Level H2 Chemistry Video Lessons
Chemistry Guru, Singapore's reputable JC Chemistry tuition centre, has a huge collection of short video lessons that targets important H2 Chemistry concepts and common questions.
Join my 19,000 subscribers on my YouTube Channel for new video lessons every week!
Follow me on Instagram for H2 Chemistry videos and (not so funny) memes!
You can also view other A Level H2 Chemistry videos here at my website.
Need an experienced tutor to make Chemistry simpler for you?
My weekly classes in Singapore are ideal for students who prefer a more structured program.
Build a strong foundation and ace your exams!
Sign up now for a trial lesson at $50only!
Check out our Chemistry tuition class timing and topics covered for our popular JC1 Classes and JC2 Classes at Bishan, Singapore.
Online lessons are also available! Learn H2 Chemistry anytime, anywhere at 50% of the cost of conventional class tuition.
Find out more information about our online tuition.
