Ideal Gas Law and Applications
In the topic of Gaseous State we have a few types of calculation questions that require manipulation of the Ideal Gas equation into various forms for easy application.
In a previous video we have discussed how to use Ideal Gas equation in graph sketching.
Before we do that let's do a recap on the Ideal Gas Equation, PV = nRT.
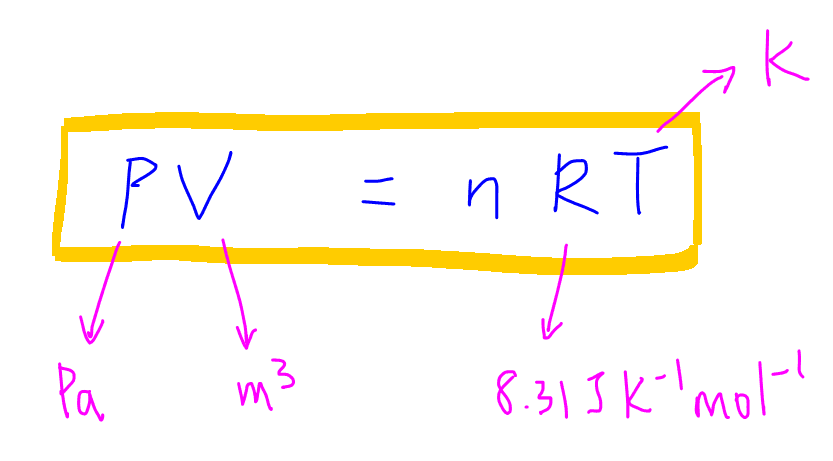
where:
P is pressure in Pa,
V is volume in m3,
n is moles of gas,
R is gas constant 8.31 J K-1 mol-1, and
T is temperature in K.
When we are using the ideal gas equation itself, remember that all the terms have to be in SI units.
Let's move on to the 3 applications that we will be discussing:
1. Boyle's Law at Constant Temperature
Usually the question will give us a fixed mass of gas hence moles of gas will be constant.
There will be a before scenario where pressure and volume are given, and when the pressure (or volume) changes at constant temperature, we are required to determine the new volume (or pressure) in the after scenario.
We can start with the Ideal Gas equation and write out the variable terms on the left hand side of equation and put the constant terms on the right hand side of equation.
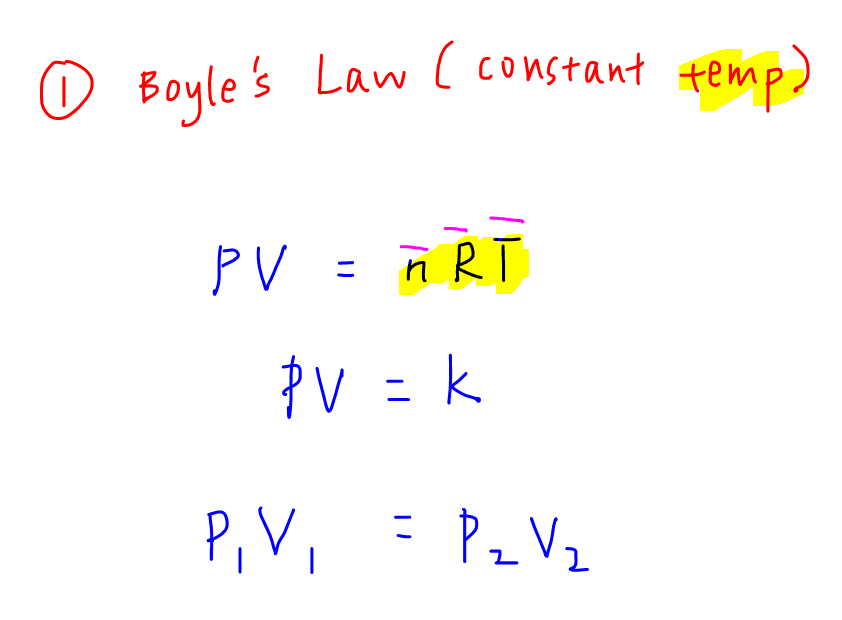
In this case at constant temp, PV is a constant hence P1V1 = P2V2, where P1 and V1 are the pressure and volume in Scenario 1 and P2 and V2 are the pressure and volume in Scenario 2.
Usually there is only 1 unknown and we can use the equation to solve for that unknown.
2. Charle's Law at Constant Pressure
We can do a similar rearrangement of the Ideal Gas equation given pressure is a constant.
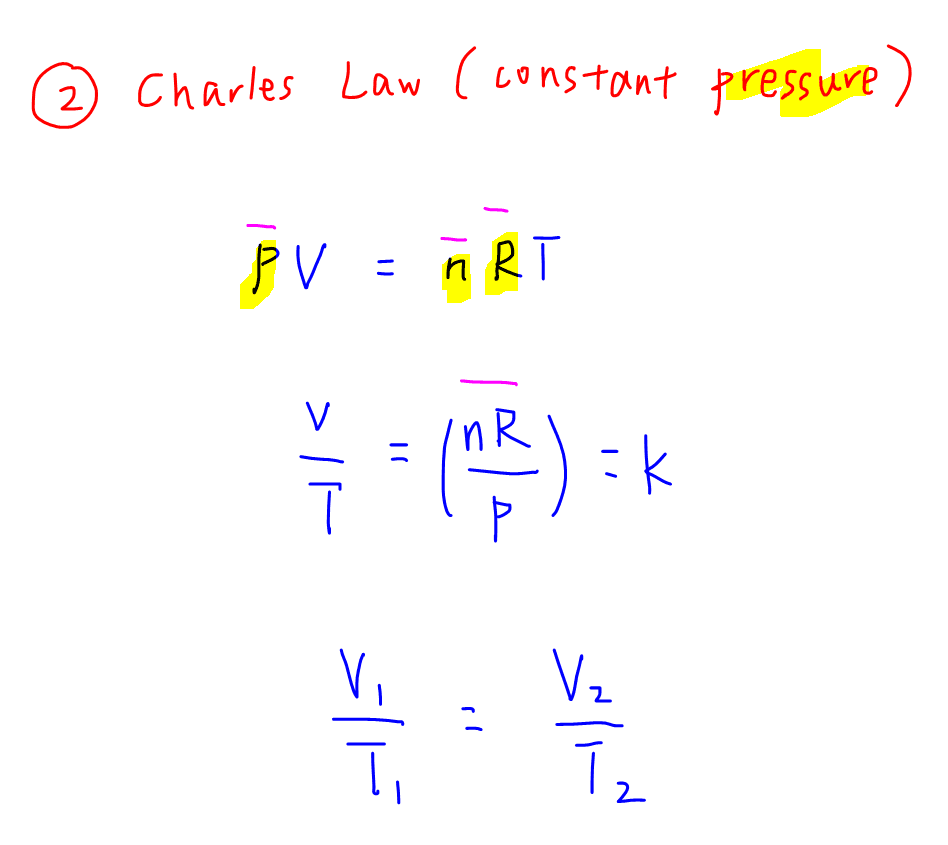
Then we can use V1 / T1 = V2 / T2 to solve for an unknown volume or temperature for a before-and-after scenario type of question.
3. Determine Final Pressure on Mixing 2 Gases at Constant Temperature
Sometimes two different non-reacting gases are mixed and we are required to determine the final pressure.
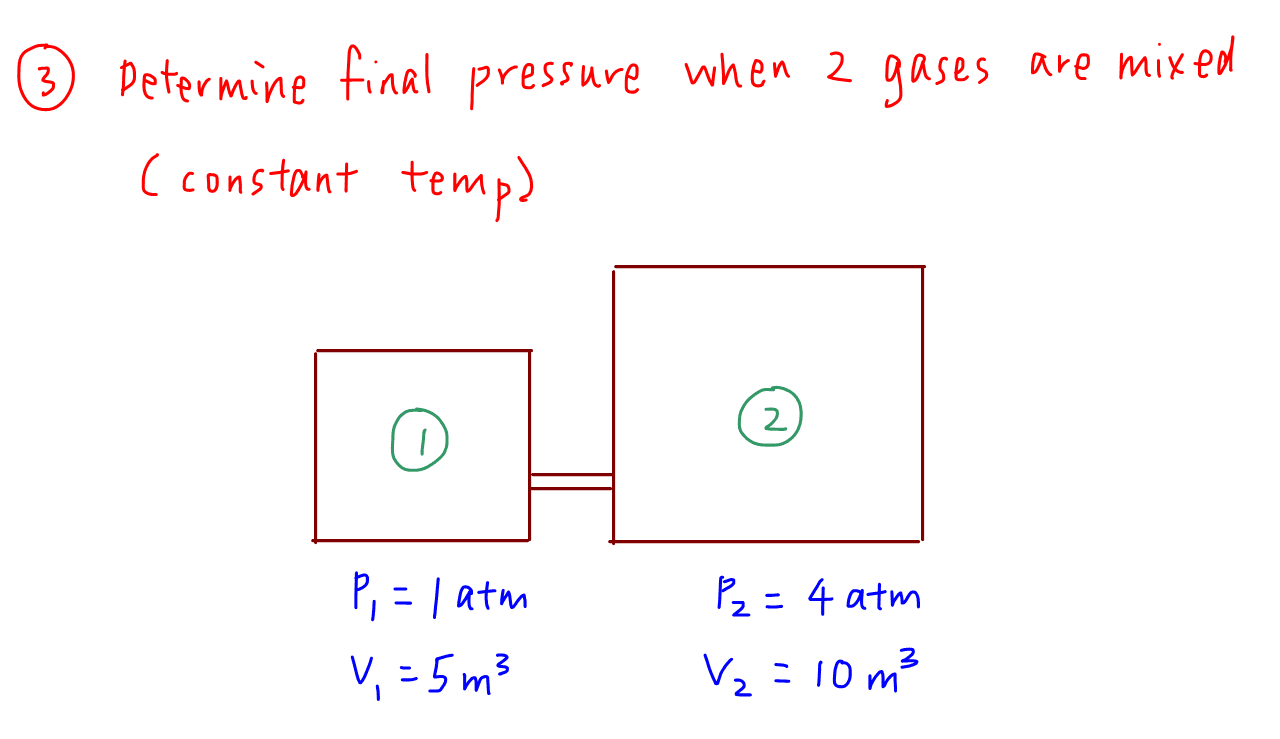
The formula is not difficult to derive so let's go through that here.
When 2 gases are mixed, the total number of moles is just the summation of moles of the first gas and the moles of the second gas, ie:
nT = n1 + n2
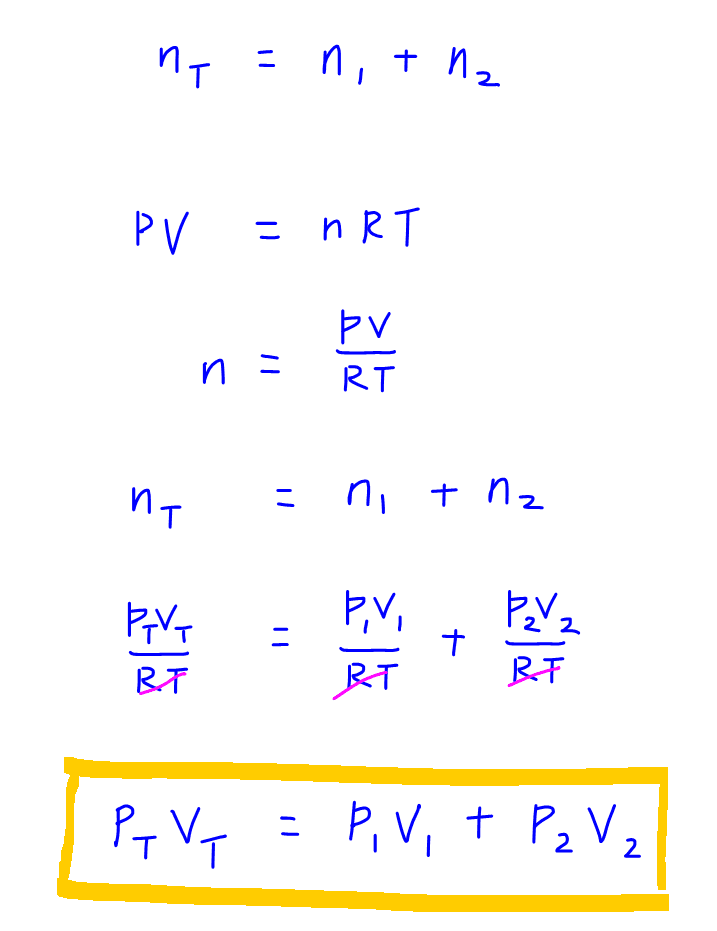
We can write moles with respect to the other terms in Ideal Gas equation and do some rearrangement to get the final equation that is very simple to apply, ie:
PTVT = P1V1 + P2V2
We can use the equation to solve for this simple exercise to determine the final pressure to be 3 atm.

For the detailed step-by-step discussion on applications of Ideal Gas equation, check out this video!
Topic: Gaseous State, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
