2019 P1 Q3 - Identify Elements via Ionisation Energy Trends
Let Chemistry Guru, Singapore's renowned JC Chemistry tuition centre, guide you through question 3 for 2019 A Levels H2 Chemistry Paper 1.
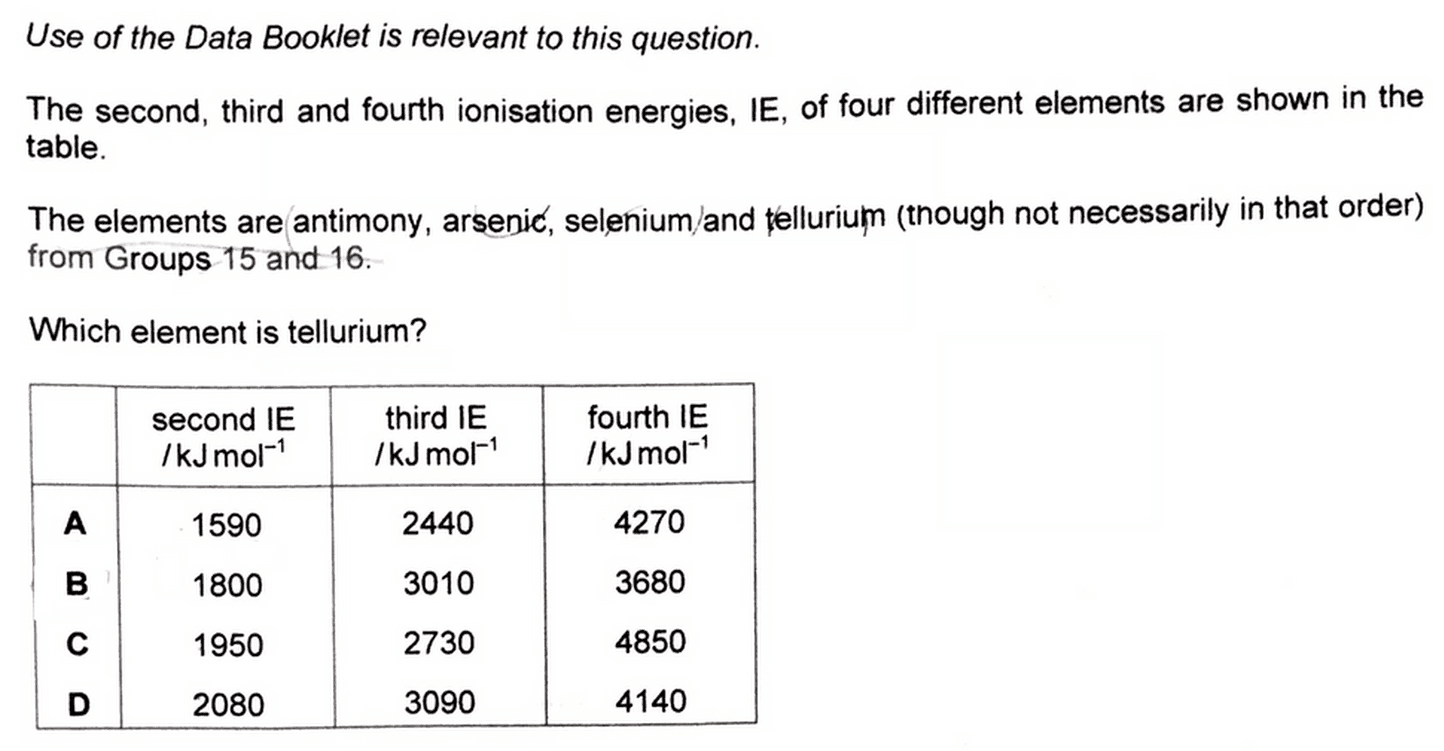
We are required to identify tellurium out of the rest of the elements given.
1. Identify Group 15 and 16 Elements
First we need to figure out the ionisation energy trends that is given in the question.
By writing out the electronic configuration, we can deduce the subshell from which the electrons are removed.
For Group 15 elements, the first 3 electrons are removed from np subshell, and the fourth electron is removed from ns subshell.
For Group 16 elements, the first 4 electrons are all removed from np subshell.

So for Group 15 elements, there is a change in subshell from 3rd to 4th ionisation.
Electrons in ns subshell are more stable as compared to np subshell and hence require significantly more energy to remove.
Therefore we will expect a significant jump in third to fourth IE for Group 15 elements.
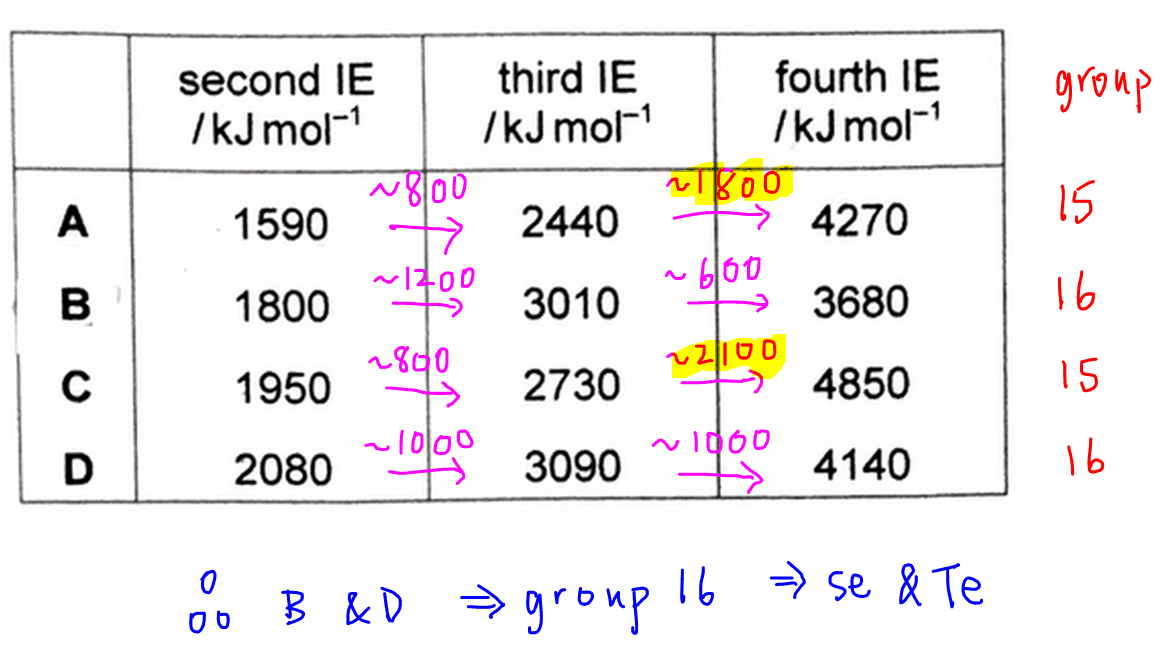
There is a significant jump from third IE to fourth IE for elements A and C.
Hence A and C are from group 15, and B and D are from group 16.
Remember we are interested in identifying tellurium which is in group 16, so we will eliminate options A and C.
2. Identify Tellurium
Comparing tellurium and selenium, Te is further down the group.
This means Te has more inner principal quantum shells and has greater shielding effect.
The valence electrons are also further away from the nucleus, so it requires less energy to remove electrons from Te.
Therefore we will expect Te to have a lower second, third and fourth IE as compared to Se.
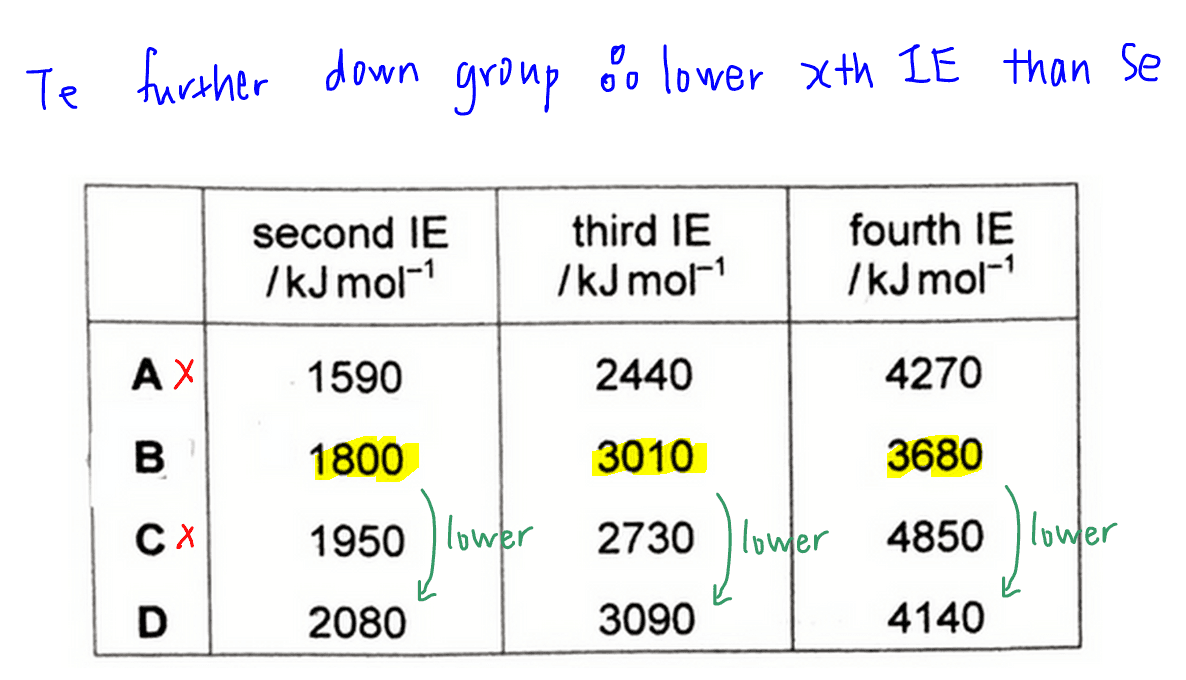
Comparing the IE trends of B and D, we can clearly see that B has lower second, third and fourth ionisation energies than D.
Hence B will be tellurium, and the answer to this question will be option B.
Topic: Atomic Structure, Physical Chemistry, A Level Chemistry, Singapore
Back to list of questions for 2019 A Level H2 Chemistry Paper 1
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top H2 Chemistry tuition choice since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or online tuition classes!
