Increase Solubility of Sparingly Soluble Salts
Let's consider how to manipulate solubility of sparingly soluble salts.
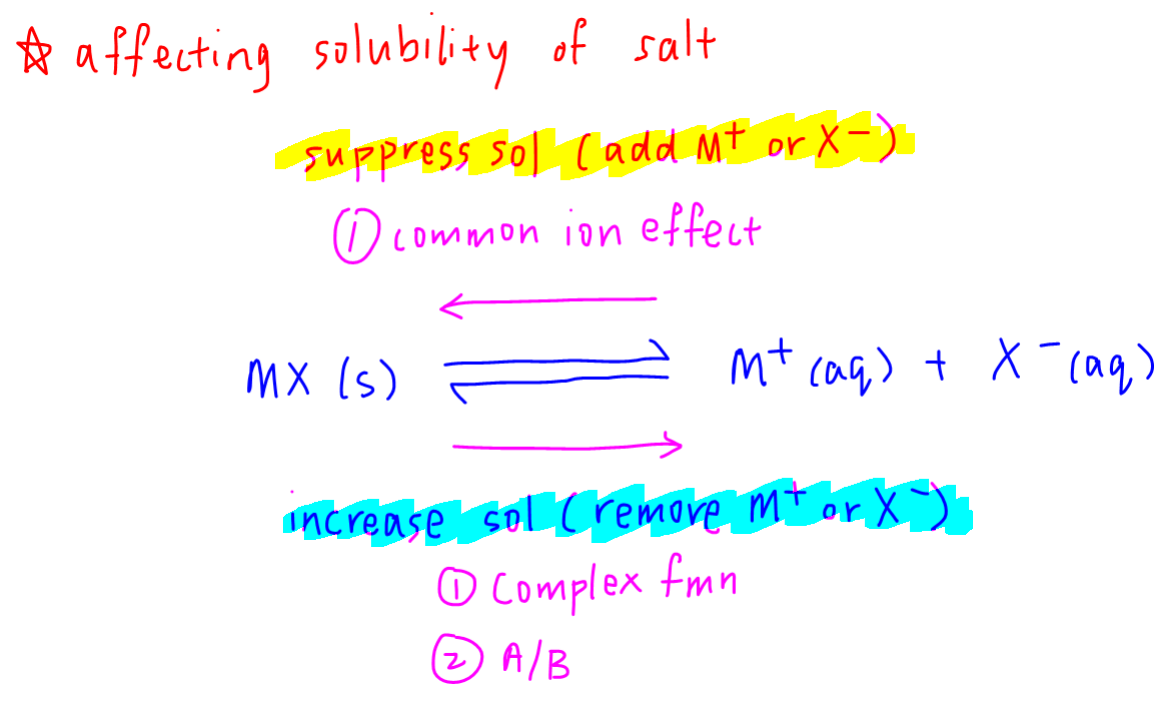
Under the concept of common ion effect, we have learnt explicitly that solubility of salts can be suppressed when a common cation or anion is added.
Check out this video lesson to learn all about common ion effect.
We can also increase solubility of salt via the following 2 methods.
1. Complex ion formation
2. Acid base reaction
Let's consider each one in detail.
1. Complex ion formation
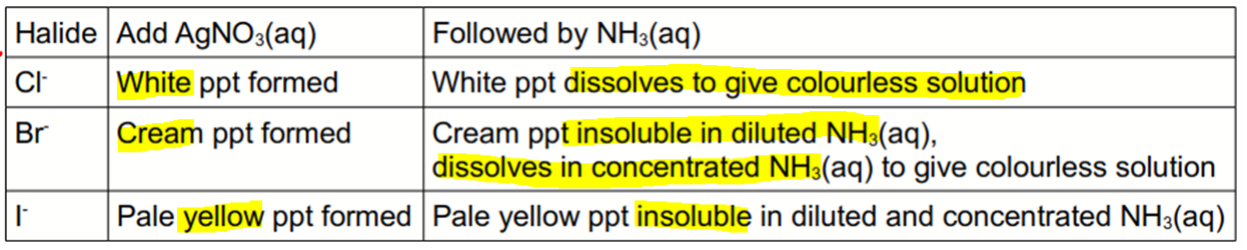
We can differentiate aqueous halides by adding silver nitrate followed by aqueous ammonia.
The observations are as follows:
When AgNO3 is added, the ionic product of silver halide is much higher than its solubility product since silver halides are sparingly soluble with very low Ksp values.
Hence silver halide precipitate is formed.

When aqueous ammonia is subsequently added, diammine silver complex is formed which is very stable.

This decreases the concentration of Ag+(aq) which will shift the position of equilibrium for the dissociation of silver halide towards the right hand side.
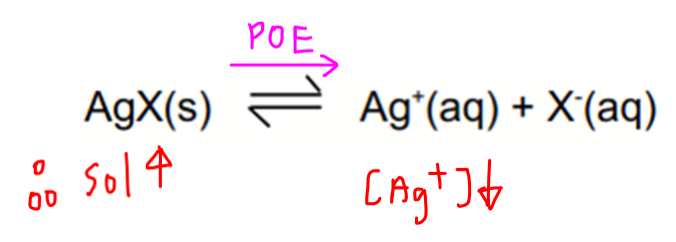
Hence more silver halide will dissolve and its solubility increases.
AgCl is the most soluble and requires only dilute ammonia to dissolve it.
AgBr is less soluble so will need concentrated ammonia to dissolve.
AgI is the least soluble and will not dissolve even with concentrated ammonia.
2. Acid base reaction
If the sparingly soluble salts are carbonates or hydroxides, we can add acid to react with the anions and reduce their concentrations.


In both cases concentration of carbonate and hydroxide decrease, position of equilibrium for dissociation of salt shifts to the right, more salt dissolves and solubility increases.
Topic: Solubility Product, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's top JC Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my JC Chemistry Tuition classes at Bishan or on-demand video lessons!
