Intramolecular versus Intermolecular Hydrogen Bond
Let Chemistry Guru, Singapore's top JC Chemistry tuition centre, guide you through the melting points of some isomers of hydroxybenzoic acid.

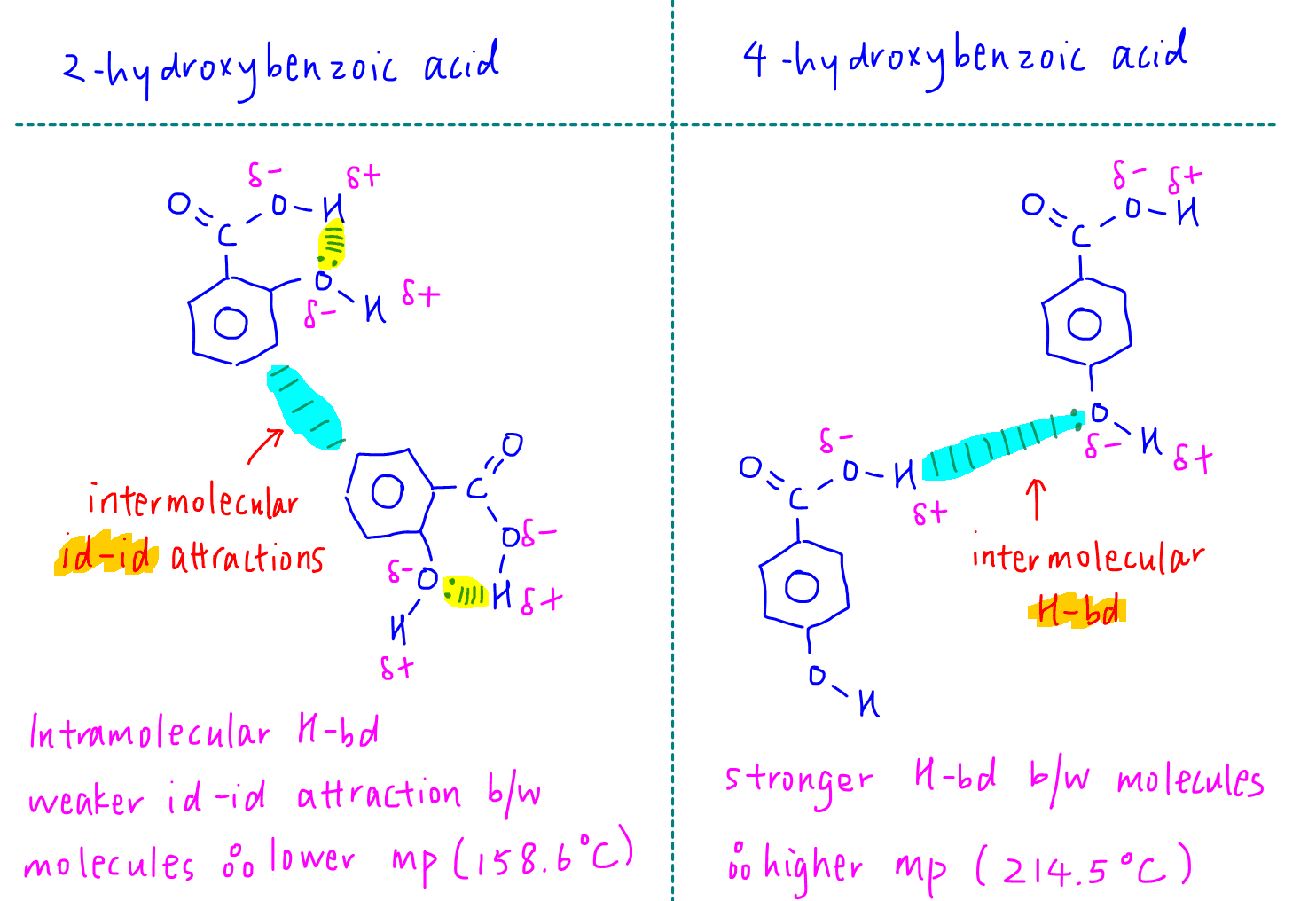
We notice that the melting point is lowest (158.6oC) for 2-hydroxybenzoic acid, where the alcohol group is closest to the acid group.
Melting point is highest (214.5oC) for 4-hydroxybenzoic acid, where the alcohol group is farthest away from the acid group.
All isomers of hydroxybenzoic acid can form hydrogen bonds due to the presence of O-H bonds.
The position of the -OH group with respect to the -COOH group has an effect on the extensiveness of hydrogen bonds between molecules, and hence their melting points.
1. Intermolecular Hydrogen Bonds in 4-hydroxybenzoic acid
The -OH and -COOH groups are pointing opposite each other and are unable to interact with each other within the molecule.
Therefore both groups can be used for hydrogen bonds between molecules.

This results in more extensive hydrogen bonds and higher melting point for 4-hydroxybenzoic acid.
2. Intramolecular Hydrogen Bonds in 2-hydroxybenzoic acid
The -OH and -COOH groups are next to each other and can form hydrogen bond within the molecule.

Since hydrogen bonds are used up for intramolecular interaction, the intermolecular forces between 2-hydroxybenzoic acid molecules will be weaker instantaneous dipole-induced dipole (id-id) attractions, or dispersion forces or Van der Waals forces.
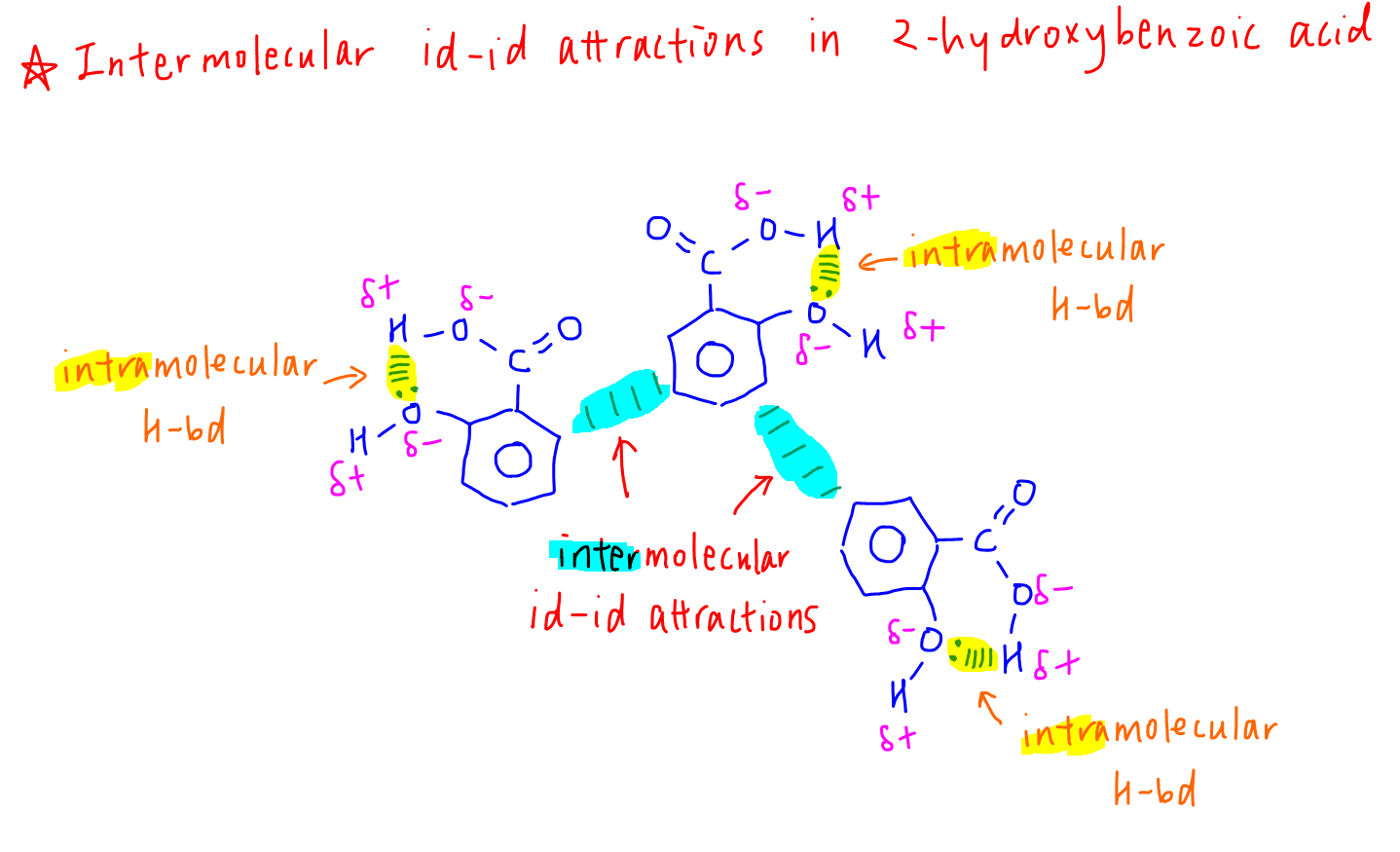
Hence the melting point of 2-hydroxybenzoic acid is lower.
In summary this is the comparison and reason why intramolecular hydrogen bond in 2-hydroxybenzoic acid lowers its melting point as compared to 4-hydroxybenzoic acid.
Topic: Intermolecular Forces, Physical Chemistry, A Level Chemistry, Singapore
Back to other previous Physical Chemistry Video Lessons.
Found this A Level Chemistry video useful?
This free chemistry video lesson is brought to you by Chemistry Guru, Singapore's reputable A Level Chemistry tuition centre since 2010.
Please like this video and share it with your friends!
Join my 19,000 subscribers on my YouTube Channel for new A Level Chemistry video lessons every week.
Check out other A Level Chemistry Video Lessons here!
Need an experienced tutor to make Chemistry simpler for you?
Do consider signing up for my A Level H2 Chemistry Tuition classes at Bishan or online tuition classes!
